Abstract
Objectives: Atraumatic and complete insertion of the electrode array is a stated objective of cochlear implant surgery. However, it is known that obstructions within the cochlea such as new bone formation, cochlear otosclerosis, temporal bone fracture, and cochlear anomalies may limit the depth of insertion of the electrode array. In addition, even among patients without obvious clinical or radiographic indicators of obstruction, incomplete insertion may occur. The current study is a histopathologic evaluation of possible sources of resistance to insertion of the electrode array using the temporal bone collection of the Massachusetts Eye and Ear Infirmary. Methods: Forty temporal bones from patients who in life had undergone cochlear implantation were evaluated. Temporal bones were removed at autopsy and fixed and prepared for histologic study by standard techniques. Specimens were then serially sectioned and reconstructed by 2-dimensional methods. Two electrode metrics were determined for each bone: the inserted length (IL: the distance measured from the cochleostomy site to the apical tip of the electrode) and the active electrode length (AEL: the distance between the most basal and most apical electrodes on the electrode array). The ratio of these two metrics (IL/AEL) was used to split the temporal bones into two groups: those with incomplete insertion (n = 27, IL/AEL <1.0) and those with complete insertion (n = 13, IL/AEL ≧1.0). Seven possible histopathologic indicators of resistance to insertion of the electrode due to contact with the basilar membrane, osseous spiral lamina and/or spiral ligament were evaluated by analysis of serial sections from the temporal bones along the course of the electrode tracks. Results: Obvious obstruction by abnormal intracochlear bone or soft tissue accounted for only 6 (22%) of the 27 partial insertions. Of the remaining 21 bones with incomplete insertions and 13 bones with complete insertions, dissection of the spiral ligament to the lateral cochlear wall was the only histopathologic indicator of insertion resistance identified with significantly higher frequency in the partial-insertion bones than in the complete-insertion bones (p = 0.003). An observed trend for the percentage of complete insertions to decrease with the number of times the electrode penetrated the basilar membrane did not reach significance. In the bones without an obvious obstruction, the most frequently observed indicator of insertion resistance was dissection of the spiral ligament (with no contact of the lateral cochlear wall) identified in 67% (14/21) of partial-insertion bones and in 92% (12/13) of complete-insertion bones. Conclusion: These results are consistent with the view that (1) electrode contact with cochlear structures resulting in observable trauma to the basilar membrane, osseous spiral lamina and/or spiral ligament does not necessarily impact the likelihood of complete insertion of the electrode array and (2) once contact trauma to the spiral ligament reaches the point of dissection to the cochlear wall, the likelihood of incomplete insertion increases dramatically.
Introduction
Atraumatic and complete insertion of the cochlear implant electrode array is a stated goal of implantation surgery [Nadol, 1984; Kennedy, 1987; Welling et al., 1993]. Atraumatic surgery is more likely to preserve existing acoustic function and allow electroacoustic stimulation in some patients [Nadol et al., 2001]. In addition, there is some evidence that complete insertion of the electrode array results in better performance, as measured by word comprehension scores during life [Skinner et al., 2002; Yukawa et al., 2004].
Incomplete insertion is well known in cases with deafness caused by meningitis in which labyrinthitis ossificans may narrow or occlude the cochlear scalae [Gantz et al., 1988; Rauch et al., 1997]. The bony dysplasia of otosclerosis may also mechanically interfere with cochlear implantation [Fayad et al., 1990]. Anomalies of the inner ear are also a common cause of incomplete insertion, particularly in the pediatric population [Zheng et al., 2002]. In addition, in cases in which there is no obvious clinical or radiographic evidence of obstruction, incomplete insertion is not uncommon [Hartrampf et al., 1995; Skinner et al., 2002; Khan et al., 2005]. This histopathologic study of 40 temporal bones from 38 human subjects who in life underwent cochlear implantation evaluates possible causes of incomplete insertion of the electrode array.
Materials and Methods
Temporal bones were removed at autopsy, fixed in 10% buffered formalin, and decalcified in ethylenediaminetetraacetic acid. Those specimens in which the electrode array was left in situ were postfixed in 2% osmium tetroxide. All specimens were dehydrated in graded alcohols. The specimens in which the electrode array was left in situ were exchanged with propylene oxide and then embedded in Araldite. Specimens in which the electrode array had been removed during fixation were embedded in celloidin. The embedded sections were serially sectioned in the horizontal (axial) plane at an average thickness of 20 µm. Specimens embedded in Araldite with the electrode array in situ were sectioned by a technique previously described [Nadol et al., 1994]. Every tenth section of specimens embedded in Araldite was left unstained or stained in toluidine blue O before mounting on a glass slide. Every tenth section of specimens embedded in celloidin was stained with hematoxylin and eosin before mounting. The semiserial sections were reconstructed by conventional 2-dimensional methods [Guild, 1921; Nadol, 1988; Schuknecht, 1993]. The depth of insertion of the cochlear implant electrode was evaluated by direct microscopic determination of the most apical section in which the electrode was visible or, in specimens in which the electrode had been removed prior to sectioning, by determining the most apical section of the cochlea with the lumen in new fibrotic and/or bony tissue that marks the electrode track in each bone. Two metrics associated with the implanted electrode were defined: (1) the inserted electrode length (IL: the distance measured from the surgical cochleostomy in the basal turn to the apical tip of the electrode array) and (2) the active electrode length (AEL: the distance between the most apical and the most basal electrodes on the electrode array). The AEL as specified by the manufacturer for each electrode was Nucleus 24R: 11.73 mm; Nucleus 24M, Nucleus 24RST and Nucleus 22: 15.75 mm; Ineraid/Richards/Symbion: 20 mm; Advanced Bionics Clarion: 15 mm, and Med-El: 19.6 mm. The ratio of IL to AEL was calculated for each bone. Incomplete insertion was defined by an IL/AEL ratio of less than 1.0. Complete insertion was defined as an IL/AEL ratio equal to or greater than 1.0.
The semiserial sections were evaluated for evidence of the electrode array encountering resistance as it was inserted. In addition to abnormal intrascalar bone and soft tissue blocking advancement of an electrode array, contact of the array with normal cochlear structures can also result in resistance to insertion. Table 1 lists possible sources of such contact resistance and the histopathologic indicators used in this study to identify locations where an electrode array made significant contact with the source during insertion.
Results
Demographic data for the 38 patients are presented in table 2. The causes of deafness included meningogenic labyrinthitis (n = 7), otosclerosis (n = 4), temporal bone fracture (n = 3), sudden sensorineural hearing loss (n = 3), Ménière’s disease (n = 2), genetically determined hearing loss (n = 2), aminoglycoside ototoxicity (n = 2), chronic otitis media (n = 1), mumps and/or chemotherapy (n = 1), acoustic schwannoma (n = 1), autoimmune sensorineural hearing loss (n = 1), superficial hemosiderosis (n = 1), acoustic trauma (n = 1) and unknown (n = 9). The age of the patients ranged from 6 to 94 years (mean: 70). The specimens were obtained from patients who had been implanted between 1 and 17 years (mean: 8.8 years) before death.
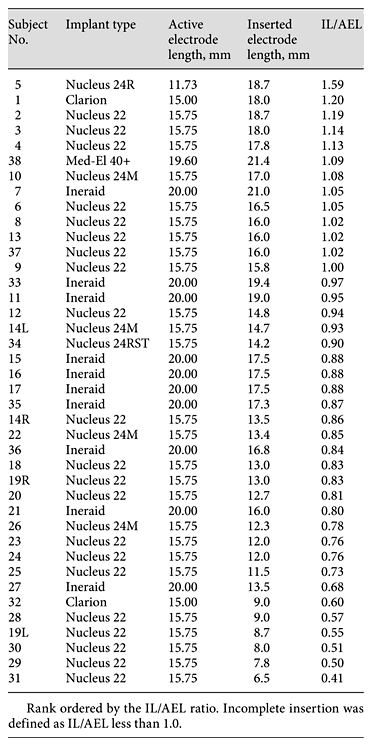
Table 3 lists the implant type, AEL, IL and the IL/AEL ratio for each subject in decreasing order of IL/AEL. Given the nature of this study, it is not surprising that few of the implants represent the latest models offered by today’s manufacturers. Of the 40 implants, 36 were relatively stiff, straight electrode systems [26 fully banded Nucleus (model 22 or model 24) electrode systems and 10 Ineraid], 1 was a more modern, straight electrode array (Med-El Combi 40+), 2 were older precurved arrays (Clarion) and 1 a relatively recent precurved design (Nucleus 24R Contour). The IL ranged from 6.5 to 21.4 mm (mean = 14.8 mm) and the IL/AEL ratio ranged from 0.41 to 1.59 (mean = 0.89). Complete insertion (IL/AEL ≥1) occurred in 13 temporal bones, and incomplete insertion occurred in 27 temporal bones.
Among the 40 ears, the electrode array first entered the cochlea via the scala tympani in 21 specimens, via the scala vestibuli in 16 specimens and both scalae in 1 case (subject 32). In 2 specimens, 1 from subject 19L in which the cochlear fluid spaces were replaced by a foreign body giant cell granuloma, and 1 from subject 30 in which a drill-out passed through the modiolus, the scala into which the electrode first entered the cochlea could not be determined. The scalar entry point did not show a significant effect on complete versus incomplete insertion (contingency analysis, d.f. = 3, χ2 = 1.63, p = 0.65).
Obvious Obstructions
Of the 27 bones with incomplete electrode insertions, 6 (subjects 19L, 24, 25, 28, 30 and 32) were found to have obvious obstructions that accounted for the partial insertion. Soft tissue obstructed insertion in 2 cases: a foreign body granuloma (presumably elicited by the first implantation of this ear) within the cochlea of subject 19L (fig. 1) and a preexistent intralabyrinthine schwannoma involving the basal turn of subject 24’s cochlea (fig. 2). Bony obstructions accounted for incomplete insertions of the remaining 4 subjects. In 2 specimens (subjects 25 and 30), the progression of the electrode array was obstructed by bone due to labyrinthitis ossificans (fig. 3, 4). In subject 28, the electrode was entrapped by bony spicules at the cochleostomy site (fig. 5).
Obstruction by giant cell granuloma. Subject 19L suffered genetically determined deafness. Because of poor performance after primary cochlear implantation of the left ear, he underwent explantation and reimplantation. An intense necrotizing granulomatous process surrounded the electrode track from the mastoid into the cochlea. The granulomatous process filled the entire cochlea, particularly in the basal turn and involved the modiolus. The track of the electrode array was present outside the basal turn of the cochlea (arrow) (hematoxylin-eosin stain).
Obstruction by giant cell granuloma. Subject 19L suffered genetically determined deafness. Because of poor performance after primary cochlear implantation of the left ear, he underwent explantation and reimplantation. An intense necrotizing granulomatous process surrounded the electrode track from the mastoid into the cochlea. The granulomatous process filled the entire cochlea, particularly in the basal turn and involved the modiolus. The track of the electrode array was present outside the basal turn of the cochlea (arrow) (hematoxylin-eosin stain).
Obstruction by soft tissue. An extensive intralabyrinthine schwannoma involved the vestibular system and basal turn of the cochlea of the right ear (subject 24). The track of the electrode array (arrow) was present in the basal turn of the cochlea (hematoxylin-eosin stain).
Obstruction by soft tissue. An extensive intralabyrinthine schwannoma involved the vestibular system and basal turn of the cochlea of the right ear (subject 24). The track of the electrode array (arrow) was present in the basal turn of the cochlea (hematoxylin-eosin stain).
Obstructing bone at the tip of the electrode. Subject 25 suffered streptococcal meningitis at the age of 18 months. Two months later, a CT scan of the temporal bone showed no evidence of labyrinthitis ossificans. However, a repeat CT scan done 2 months thereafter showed new bone growth in both cochleae. Cochlear implantation was done at the age of 2 years in the left ear. There was extensive labyrinthitis ossificans throughout the cochlea. New bone was identified beyond the electrode tip (unstained).
Obstructing bone at the tip of the electrode. Subject 25 suffered streptococcal meningitis at the age of 18 months. Two months later, a CT scan of the temporal bone showed no evidence of labyrinthitis ossificans. However, a repeat CT scan done 2 months thereafter showed new bone growth in both cochleae. Cochlear implantation was done at the age of 2 years in the left ear. There was extensive labyrinthitis ossificans throughout the cochlea. New bone was identified beyond the electrode tip (unstained).
Surgical ‘drill-out’ through modiolus. Subject 30 suffered 3 episodes of meningitis due to head trauma and lost all hearing in both ears at age 38. Part of the modiolus, the superior aspect of the basal turn, and the inferior aspect of the apical turn were drilled to achieve this implantation of the right ear. The electrode track (CI) crossed the modiolus of the middle turn. The remaining cochlea is nearly completely replaced by labyrinthitis ossificans (hematoxylin-eosin stain).
Surgical ‘drill-out’ through modiolus. Subject 30 suffered 3 episodes of meningitis due to head trauma and lost all hearing in both ears at age 38. Part of the modiolus, the superior aspect of the basal turn, and the inferior aspect of the apical turn were drilled to achieve this implantation of the right ear. The electrode track (CI) crossed the modiolus of the middle turn. The remaining cochlea is nearly completely replaced by labyrinthitis ossificans (hematoxylin-eosin stain).
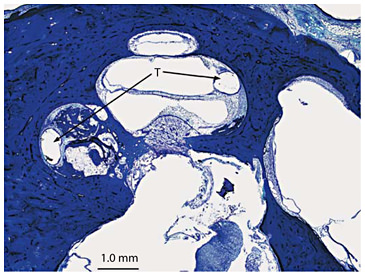
Impingement of the electrode on bony spicule. Subject 28 suffered a progressive loss of hearing in both ears of unknown cause, starting in childhood. Cochlear implantation was done in the right ear at age 77. Bony spicule (B) impinged upon the track (T) of the cochlear implant (hematoxylin-eosin stain).
Impingement of the electrode on bony spicule. Subject 28 suffered a progressive loss of hearing in both ears of unknown cause, starting in childhood. Cochlear implantation was done in the right ear at age 77. Bony spicule (B) impinged upon the track (T) of the cochlear implant (hematoxylin-eosin stain).
Histopathologic Indicators of Resistance to Electrode Insertion
The 21 bones with incomplete insertions in which no obvious intrascalar obstruction was identified and the 13 complete-insertion bones are listed in table 4 with the resistance indicators of table 1 that were identified during histopathologic examination of each bone. Examples of each histologic indicator are given in figures 6, 7, 8, 9, 10, 11, 12.
Distribution by subject (excluding subjects with obvious obstructions) of the observed indicators of an electrode encountering insertion resistance

Displacement of the basilar membrane in the ascending limb of the basal turn of the left ear in subject 14L in whom the cause of deafness was otosclerosis. The fibrous sleeve (S) that surrounded the electrode was present in the scala tympani. The basilar membrane (BM) was displaced toward the scala media (hematoxylin-eosin stain).
Displacement of the basilar membrane in the ascending limb of the basal turn of the left ear in subject 14L in whom the cause of deafness was otosclerosis. The fibrous sleeve (S) that surrounded the electrode was present in the scala tympani. The basilar membrane (BM) was displaced toward the scala media (hematoxylin-eosin stain).
Disruption of the basilar membrane in the ascending limb of the basal turn of the left ear in subject 11 in whom the cause of deafness was pneumococcal meningitis. The basilar membrane (BM) was disrupted and displaced toward the scala vestibuli by the electrode, and new bone formation had obliterated most of the fluid space of the scala tympani (unstained).
Disruption of the basilar membrane in the ascending limb of the basal turn of the left ear in subject 11 in whom the cause of deafness was pneumococcal meningitis. The basilar membrane (BM) was disrupted and displaced toward the scala vestibuli by the electrode, and new bone formation had obliterated most of the fluid space of the scala tympani (unstained).
Transscalar migration of the electrode in subject 12, who became profoundly deaf at age 59 years secondary to chronic otitis media. Explantation of a malfunctioning single channel device and reimplantation with a multichannel electrode was performed in this right ear. New bone was found in the inner ear for approximately 2 mm apical to the cochleostomy site. The electrode track (T) passed through the basilar membrane from one scala to another scala at 3 separate locations (toluidine blue O stain).
Transscalar migration of the electrode in subject 12, who became profoundly deaf at age 59 years secondary to chronic otitis media. Explantation of a malfunctioning single channel device and reimplantation with a multichannel electrode was performed in this right ear. New bone was found in the inner ear for approximately 2 mm apical to the cochleostomy site. The electrode track (T) passed through the basilar membrane from one scala to another scala at 3 separate locations (toluidine blue O stain).
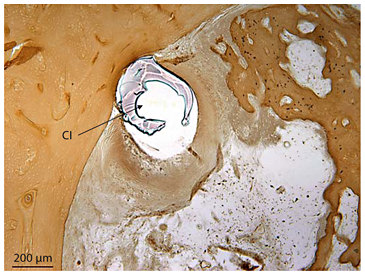
Displacement of osseous spiral lamina in the ascending limb of the basal turn of the right ear in subject 17 who became totally deaf as a complication of prolonged treatment with aminoglycoside. The electrode ball of the Ineraid cochlear implant (CI) had displaced the osseous spiral lamina (OSL) and the scala tympani was filled with new bone (toluidine blue O stain).
Displacement of osseous spiral lamina in the ascending limb of the basal turn of the right ear in subject 17 who became totally deaf as a complication of prolonged treatment with aminoglycoside. The electrode ball of the Ineraid cochlear implant (CI) had displaced the osseous spiral lamina (OSL) and the scala tympani was filled with new bone (toluidine blue O stain).
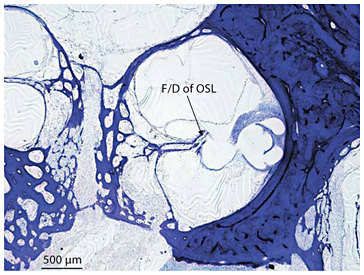
Fracture/dislocation of osseous spiral lamina in the middle turn of the right ear in subject 21 who became totally deaf secondary to bilateral sudden hearing loss with vertigo at age 20 years. Fracture and dislocation (F/D) of the osseous spiral lamina (OSL) by the electrode of an Ineraid cochlear implant was seen (toluidine blue O stain).
Fracture/dislocation of osseous spiral lamina in the middle turn of the right ear in subject 21 who became totally deaf secondary to bilateral sudden hearing loss with vertigo at age 20 years. Fracture and dislocation (F/D) of the osseous spiral lamina (OSL) by the electrode of an Ineraid cochlear implant was seen (toluidine blue O stain).
Dissection of spiral ligament in the left ear of subject 15 who had bilateral progressive sensorineural hearing loss starting in his high school years due to otosclerosis. He underwent a stapedectomy with a fat-wire prosthesis in the left ear and a stapedectomy with a Teflon wire piston in the right. There was dissection of the spiral ligament (SL) by the electrode (CI) in the descending basal turn (unstained).
Dissection of spiral ligament in the left ear of subject 15 who had bilateral progressive sensorineural hearing loss starting in his high school years due to otosclerosis. He underwent a stapedectomy with a fat-wire prosthesis in the left ear and a stapedectomy with a Teflon wire piston in the right. There was dissection of the spiral ligament (SL) by the electrode (CI) in the descending basal turn (unstained).
Dissection of spiral ligament to the bony cochlear wall in the ascending limb of the basal turn of the left ear in subject 20 who had bilateral profound hearing loss of unknown cause since childhood but in whom cochleosaccular degeneration was identified histologically. The electrode (CI) had dissected to the bony wall in the ascending basal turn, 5.5 mm from cochleostomy (unstained).
Dissection of spiral ligament to the bony cochlear wall in the ascending limb of the basal turn of the left ear in subject 20 who had bilateral profound hearing loss of unknown cause since childhood but in whom cochleosaccular degeneration was identified histologically. The electrode (CI) had dissected to the bony wall in the ascending basal turn, 5.5 mm from cochleostomy (unstained).
The indicator observed most frequently was dissection of the spiral ligament being identified in 67% of bones with incomplete insertion and in 92% of bones with complete insertion. Only 4 (12%) of the bones (subjects 5, 19R, 23, 29) were found to be free of evidence that the electrode encountered a potential source of resistance. Of these, complete insertion was achieved in subject 5 and incomplete insertion in subjects 19R, 23, and 29.
The distribution of resistance indicators between bones with complete and those with incomplete insertions are shown in table 5 for bones without obvious obstructions. Except for dissection of the spiral ligament and displacement of the osseous spiral lamina, the prevalence of each indicator was higher in the group with incomplete insertion. However, the only indicator for which the difference was significant was dissection of the spiral ligament to the bony wall (p = 0.003; Fisher’s exact test). All electrodes dissecting the spiral ligament to the bony wall were either Ineraid or fully banded Nucleus arrays; the stiffest of the straight arrays represented in this study. Because of the small number (4/40) of electrodes that are not Ineraid or fully banded Nucleus arrays, it is not possible to use our sample to test whether the newer electrode systems are less likely to produce this type of trauma. We analyzed the subgroup of the Ineraid and banded Nucleus arrays and found the stiffer Ineraid more likely to penetrate the spiral ligament to the bony wall (χ2 = 6.8; d.f. = 1; p = 0.009).
Percentage of bones (without obvious obstructions) in which the listed indicator of an electrode encountering insertion resistance is identified

Table 5 shows that the frequency of the electrode penetrating the basilar membrane was 71% in the group with incomplete insertion as compared to 38% of the bones with complete insertions. While this difference did not reach significance (p = 0.06; Fisher’s exact test), we did observe a trend for the percentage of complete insertions to decrease as a function of the number of times the electrode crossed from one scala to another. The 34 bones of table 4 were partitioned into five groups depending on the number of crossings from one scala to another. The percentage of complete insertions is plotted by group (number of basilar membrane crossings) in figure 13a. While a tendency for the percentage of complete insertions to decrease with increasing number of crossings is evident in figure 13a, a χ2 test of independence between complete insertion and number of crossings did not demonstrate a significant association between these two variables (χ2 = 5.7; d.f. = 4; p = 0.22).
a The 34 bones without an obvious obstruction were grouped by the number of times the electrode crossed (penetrated) the basilar membrane. The percentage of bones with complete electrode insertions is plotted as a function of the number of crossings. b The same 34 bones are grouped by the number of resistance indicators identified by histologic examination. The percentage of bones with complete electrode insertions is plotted as a function of the number of indicators. a, b The number at the base of each bar documents the number of bones in each grouping.
a The 34 bones without an obvious obstruction were grouped by the number of times the electrode crossed (penetrated) the basilar membrane. The percentage of bones with complete electrode insertions is plotted as a function of the number of crossings. b The same 34 bones are grouped by the number of resistance indicators identified by histologic examination. The percentage of bones with complete electrode insertions is plotted as a function of the number of indicators. a, b The number at the base of each bar documents the number of bones in each grouping.
The bones were also grouped by the total number of resistance indicators identified in each (table 4). Figure 13b plots the percentage of complete insertions by group (number of indicators) and does not show a strong tendency for complete insertions to decrease as the number of resistance indicators increased. A χ2 test of independence between complete insertion and number of indicators confirmed little association between the two variables (χ2 = 6.8; d.f. = 7; p = 0.45).
Discussion
Atraumatic and complete insertion of the electrode array is a stated goal of cochlear implant surgery in order to achieve better word recognition [Donnely et al., 1995], to reduce the biologic response to the implant [Nadol and Eddington, 2004], and to preserve residual acoustic hearing [Rossi and Bisetti, 1998]. Incomplete insertion of the electrode in some cases may be caused by intrascalar luminal obstruction by bone, such as in labyrinthitis ossificans, otosclerosis and temporal bone fracture, or by soft tissue.
Labyrinthitis Ossificans
Meningogenic labyrinthitis is a common cause of acquired profound sensorineural hearing loss and frequently causes labyrinthitis ossificans [Rauch et al., 1997]. In the current study, there were 7 subjects in whom meningogenic labyrinthitis was the cause of deafness (subjects 7, 11, 18, 25, 27, 30 and 37). Of these, a ‘drill-out’ was required in 2 subjects, 1 in the lower basal turn (subject 25, fig. 3) and 1 in the middle turn (subject 30, fig. 4), and incomplete insertion occurred in both.
Otosclerosis
The second most common site of predilection for otosclerotic involvement of the cochlear capsule is the round window [Schuknecht and Barber, 1985]. In addition, otosclerosis may cause at least partial obstruction of the scala tympani [Fayad et al., 1990]. In the current study, otosclerosis was the cause of deafness in 5 of the 40 temporal bones. Incomplete insertion occurred in 4 ears (14L, 14R, 15 and 36). In the 4 temporal bones with otosclerosis in which incomplete insertion occurred, there was no evidence of intrascalar obstruction caused by otosclerosis. Rather, in these cases there were 1 or more other findings that possibly resulted in incomplete insertion (table 5).
In 1 case of complete insertion (subject 10), extensive otosclerotic obstruction of the distal 3 mm of the basal turn was identified. After drilling this away, the cochlear implant array was fully inserted without difficulty.
Temporal Bone Fracture
Fracture dislocation of the cochlear capsule may result in labyrinthitis ossificans or distortion of the cochlear lumen. Camilleri et al. [1999] described 7 cases of profound sensorineural hearing loss following unilateral or bilateral temporal bone fracture in which cochlear implantation was performed. New bone formation was described in 2 cases at the anterior end of the basal turn and in a third case where there was total obliteration of the scala tympani. Of the 40 temporal bones in the current study, the cause of deafness was temporal bone fracture in 3 (subjects 2, 3 and 4), and the insertion was complete in all 3 cases despite a complex transverse fracture through the basal, middle and apical turn in 1 case (subject 4), and osteoid and new bone formation was present in the scala vestibuli and scala tympani in the ascending limb of the basal turn in 2 cases (subjects 2 and 3).
Impingement on Bone at Cochleostomy
In subject 28, who experienced a progressive loss of hearing in both ears of unknown cause, incomplete insertion was apparently caused by impingement of the cochlear implant electrode on a bony spicule at the cochleostomy site (fig. 5).
Soft Tissue Obstruction
There were 2 temporal bones in the current study (subjects 19L, 24) in which soft tissue in the inner ear seemed to be the cause of incomplete insertion. In subject 24, a left acoustic neuroma of the cerebellopontine angle and internal auditory canal measuring approximately 3 cm in diameter was partially removed by a suboccipital craniotomy with decompression of the internal canal at age 48 years in order to preserve hearing in an only hearing ear. However, a progressive loss of hearing occurred, and at age 50 the patient underwent left cochlear implantation with incomplete insertion. Postmortem histopathology of the left temporal bone identified a vast intracochlear extension of the schwannoma as the probable cause of incomplete insertion (fig. 2). The presence of the schwannoma in the basal turn at the time of cochlear implant was noted visually and confirmed by biopsy. In case 19L, the implantation was done following explantation of a poorly functioning previously implanted electrode. The cause of the incomplete insertion on histologic study seemed to be a granulomatous foreign body process filling the entire cochlea (fig. 1). This case was previously published in detail [Nadol et al., 2008]. In this case, both ears had been implanted and both cochleae were infiltrated with a foreign body granulomatous reaction. By clinical history, the benefit provided by the opposite (right) reimplantation began to decrease within 1 year of implantation which pathologically was attributable to the foreign body reaction. On the left, the revision implantation was done 14 months after the first implantation and hence it is reasonable to assume that the foreign body reaction demonstrated postmortem had begun within that 14-month interval between primary and revision implant surgery on the left.
Other Causes of Incomplete Insertion
In previously reported series, the depth of insertion has varied considerably. Skinner et al. [2002] reported insertion depths ranging between 11.9 and 25.9 mm in 26 patients who received a Nucleus 22 cochlear implant based on cochlear reconstruction using high-resolution spiral computer tomography. Yukawa et al. [2004] reported insertion depths ranging between 10.5 and 27 mm, based on 2-dimensional radiographs done postoperatively in 48 postlingually deafened adults implanted with either the Nucleus 22 or the Nucleus 24 cochlear implant devices. In our current study, the insertional length among 34 out of a total of 40 subjects varied between 6.5 and 21.4 mm, excluding 6 subjects (19L, 24, 25, 28, 30 and 32) in whom there was clinical or radiologic evidence of soft tissue or bony obstruction.
We hypothesize that the variable insertion depth of cochlear implants in cases where there was no obvious radiologic or clinical evidence of obstruction could be explained by impingement of the electrode on normal cochlear structures which would also result in observable trauma to the inner ear. In a study of 9 human cadaveric cochlear implants, Kennedy [1987] described insertion trauma at the spiral ligament in the area of the basal turn, elevation of the basilar membrane at the 8- to 12-mm region and embedment of the tips of the electrodes in the outer wall of the scala tympani during passage around the first turn of the cochlea. Likewise, Wardrop et al. [2005] described insertion trauma to normal cochlear structures using the Nucleus Contour electrode and the Nucleus banded electrode in 26 cadaveric specimens. The most common site of trauma was located 180° from the round window consisting of penetration of the basilar membrane or osseous spiral lamina.
In a study of human temporal bone specimens from patients who in life had undergone cochlear implantation, Nadol et al. [2001] also described the lateral cochlear wall in the ascending limb of the basal turn as the most common site of postoperative new bone formation in addition to tears in the spiral ligament, and breaks in the basilar membrane, particularly in the basal turn, as common sites of trauma. Thus, the most commonly described trauma in cochlear structures as demonstrated histologically include dissection of the spiral ligament and stria vascularis, fracture or dislocation of the osseous spiral lamina, and displacement or disruption of the basilar membrane [Kennedy, 1987; Nadol et al., 2001; Welling et al., 1993]. The results of the current study confirm previous descriptions of insertion trauma that mark potential sites of resistance to the passage of the electrode. Possible causes of incomplete insertion, based on the assumption that trauma to normal cochlear structures may indicate sites of possible resistance of advancement of the electrode, are presented in table 1.
Although the sites of trauma to the inner ear were seen both with incomplete insertion as well as complete insertion, there was a statistically higher incidence of dissection of the spiral ligament to the lateral bony wall in the 21 ears with incomplete insertion (and no obvious intrascalar obstruction) as compared to those with complete insertion (p = 0.003) (table 5). The higher incidence of other indicators of resistance in incomplete versus complete insertions did not reach statistical significance in this small cohort.
The analysis of scala vestibuli versus scala tympani in table 4 with IL/AEL showed that the difference between the mean IL/AEL ratio for bones with a scala vestibuli versus scala tympani as entrance scala is small (0.96 for scala vestibuli vs. 0.85 for scala tympani), but just significant (t = 1.7; d.f. = 35.5; p = 0.049). However, this difference does not translate into the likelihood of a complete insertion being different depending on whether the entrance scala is a scala vestibuli or scala tympani (χ2 = 1.0; d.f. = 1; p = 0.31).
We evaluated the relationship between the location of the cochleostomy site and the scala first entered by the electrode in subjects of table 4 (34 subjects). In the 14 subjects in whom the electrode entered the scala vestibuli, the cochleostomy sites were anterior and superior (n = 5), superior (n = 4), inferior (n = 4) or anterior and inferior (n = 1) to the round window. In the 20 subjects in whom the electrode entered the scala tympani, the cochleostomy sites were anterior and inferior (n = 10) or inferior (n = 9) to the round window. In 1 case (subject No. 6), the exact site of the cochleostomy could not be determined because of its large size. In these subjects, a cochleostomy anterior and inferior to the round window favored entrance of the electrode into the scala tympani. However, entrance into the scala vestibuli may still occur if deflected by new bone in the scala tympani (case No. 1) or by the angle of insertion of the electrode.
Conclusion
Of the 40 temporal bones implanted during life with intracochlear electrodes, the electrode arrays were fully inserted in 13 temporal bones and partially inserted in 27. Only 6 (22%) of the 27 bones with partially inserted electrodes showed obvious bony or soft tissue obstructions to electrode insertion. Of the remaining 21 bones with incomplete insertions and 13 bones with complete insertions, dissection of the spiral ligament to the lateral cochlear wall was the only histopathologic indicator of insertion resistance identified with significantly higher frequency in the bones with incomplete insertion as compared to those with complete insertions (p = 0.003). Dissection of the spiral ligament to the bony cochlear wall was only observed for the relatively stiff Ineraid and fully banded Nucleus arrays and was more likely with the stiffer Ineraid (p = 0.009). A trend for the percentage of complete insertions to decrease with the number of times the electrode penetrated the basilar membrane was observed but did not reach significance. In the bones without an obvious obstruction, dissection of the spiral ligament (with no contact with the lateral cochlear wall) was the most frequently observed indicator of insertion resistance being identified in 67% (14/21) of bones with incomplete insertion and in 92% (12/13) of bones with complete insertion. Taken together, these results are consistent with the view that (1) electrode contact with cochlear structures resulting in observed trauma to the basilar membrane, osseous spiral lamina and/or spiral ligament does not necessarily impact the likelihood of complete insertion of the electrode array and (2) once contact trauma to the spiral ligament reaches the point of dissection to the bony cochlear wall, the likelihood of incomplete insertion increases dramatically.
Acknowledgment
This work was supported by funding from the NIH (NIDCD) grant No. R01DC000152 Electron Microscopy of the Human Inner Ear.