Abstract
With the advent of highly sensitive and specific screening of respiratory specimens for viruses, new viruses are discovered, adding to the growing list of those associated with wheezing illness and asthma exacerbations. It is not known whether early childhood infections with these viruses cause asthma, and, if so, what exactly are the pathophysiologic mechanisms behind its development. The current consensus is that respiratory viral infection works together with allergy to produce the immune and physiologic conditions necessary for asthma diasthesis. One link between viruses and asthma may be the eosinophil, a cell that plays a prominent role in asthma and allergy, but can also be found in the body in response to viral infection. In turn, the eosinophil and its associated products may be novel therapeutic targets, or at the very least, used to elucidate the complex pathophysiologic pathways of asthma and other respiratory illnesses. Together or separately, they can be used for diagnosis, treatment and monitoring. Not only symptoms, but also the underlying disease mechanisms must be taken into consideration for the optimal care of a patient.
Introduction
There is a dire need to understand the underlying pathophysiology of asthma, including the complex genetic and environmental influences, to develop more effective treatment strategies. Though the role of respiratory viruses in asthma has been extensively studied over the past few decades, many questions remain unanswered. First, of the known viruses, which are the most likely to cause an infection associated with recurrent wheezing/asthma development? Second, do early childhood infections with respiratory viruses cause asthma or do they merely select those who are predisposed to these infections? Third, if they are a causal factor, what is the immunopathology behind it? In the following sections, the interactions between viral respiratory infections and asthma development/exacerbations will be explored, with an emphasis on those viruses associated with eosinophilia.
Infections in Infancy and Asthma Development
It is well known that a substantial portion of infants who require hospitalization for bronchiolitis develop asthma by the age of 13 years [1,2,3,4,5]. The importance of an early-life viral infection has been highlighted by studies showing that the timing of birth in relation to the winter virus season can increase the risk of developing asthma by the age of 6 years by as much as 30% [6]. The exact mechanisms and pathophysiologic pathways by which viral infection leads to asthma are not known, and those that have been proposed are subject to intense debate. Risk factors for bronchiolitis include young age (especially <6 months), small lung size and exposure to tobacco smoke [7]. Despite the uncertainty, it is generally considered that respiratory viral infection does not work alone but synergistically with respiratory allergy to produce immunologic and physiologic conditions conducive to asthma development.
Respiratory syncytial virus (RSV) has been the main focus of research, and most infants will have had at least 1 infection with RSV by the age of 3 years. This virus is the major cause of bronchiolitis in children <1 year of age; consequently, it is considered the most important respiratory tract pathogen of early childhood [8]. No direct interventional studies demonstrating a causal relationship between RSV and asthma have been published to date. However, a long-term study of over 95,000 children found that timing of birth in relation to the winter virus peak (particularly RSV) independently predicted asthma development, with the highest risk estimated for those born 121 days (approx. 4 months) before the peak [6]. In addition, the administration of anti-RSV immune globulin (palivizumab) to children at high risk of chronic airway disease improved lung function and reduced the incidence of asthma and atopy, suggesting that the prevention of RSV infection has long-term effects on respiratory and immunologic parameters relevant to asthma development [9]. In a larger more recent study, palivizumab significantly reduced recurrent wheezing [10]. However, because nearly every child has been infected with RSV at least once, other genetic, environmental and/or developmental factors must also contribute to the epidemiological link with childhood asthma [11].
A number of recent papers have highlighted a possible role of human rhinovirus (HRV) in asthma etiology. HRV is now recognized as an important cause of wheezing illness; however, it is most often found in asymptomatic infants and children [12,13]. The wide variety of illness severity may be due to either host factors (abnormal innate immune responses) [14] or virus strain (more virulent or pathogenic strains) [15]. In comparative studies, children with RV-induced bronchiolitis were older and more atopic [16] and had a more severe disease course in the acute phase [17] than children with RSV-induced bronchiolitis. In the Perth Birth Cohort study, HRV was the most common pathogen associated with an acute respiratory infection in the first year of life followed by RSV [18]. Another study found that wheezing HRV illnesses during the first 3 years of life were associated with a nearly 10-fold increase in asthma risk at the age of 6 years [19]. In contrast, several studies suggest that HRV infection is not a risk factor for asthma, but may instead reveal children predisposed to asthma due to abnormal lung physiology and/or immune responses [18,20,21].
Another virus that has been implicated as a possible cause of wheezing and asthma is human bocavirus (HBoV), a novel parvovirus first isolated in 2005 from the respiratory secretions of patients with pneumonia [22]. However, the link between HBoV and asthma, or any respiratory disease, has been complicated by the fact that it has a high rate of coinfection [23]. A recent study conducted at Inje University Sanggye Paik Hospital found an association between HBoV infection and acute wheezing in children [24]. In their epidemiological study on acute wheezing and children, HBoV (13.8%) was the third most frequently found virus after RV (33.3%) and RSV (13.8%). Another epidemiological study by Vallet et al. [25] found HBoV infection to be associated with 13% of severe asthma exacerbations in children. They suggested that HBoV could play a major role in acute exacerbations in asthmatic children.
Other viral infections during infancy and early childhood causing lower respiratory infections are also associated with recurrent wheezing and asthma development. These include parainfluenza, influenza A and human metapneumovirus [20,26,27]. In our recent study, we showed that human metapneumovirus infection was associated with recurrent wheezing in children [28].
The question of whether early childhood viral infections cause asthma, or instead cause chronic disease in those with an underlying predisposition to asthma, remains unanswered.
Viruses and Asthma Exacerbations
It has been estimated that nearly 85% of asthma exacerbations in children, and nearly 50% in adults, are a result of viral infections [29,30]. Indeed, with the advent of nucleic acid amplification testing (e.g., reverse transcription polymerase chain reaction) and immunoassays, the detection of HRV and other RNA viruses has been enhanced suggesting that HRV may be responsible for a larger proportion of exacerbations than previously thought. In a study by Rakes et al. [31], HRV was the predominant pathogen (71%) in children aged 2–16 years presenting to emergency departments with acute wheezing, while only 6% were positive for RSV. A study of 206 asthmatic children aged 3–18 years found HRV (26%) as the most common cause of exacerbation [32], while in adults, HRV was again the most common pathogen (56% of virus-positive specimens) found in asthma-related acute-care visits [33]. These studies provide strong evidence of an association between respiratory viral infection and asthma exacerbation. However, it is still unclear whether viral infection alone can exacerbate asthma, or if other factors, such as exposure to allergens [34] or air pollution [35], work synergistically to precipitate an attack.
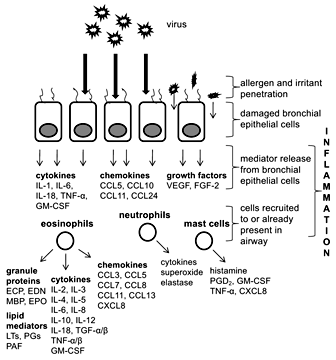
The body’s response to an invading pathogen includes the respiratory, immune and nervous systems [36]. Respiratory viral infections during infancy may have acute and long-term effects on lung and immune system development and represent a risk factor for asthma development. When bronchial epithelial cells are infected by a virus (fig. 1), they generate both local and systemic immune responses aimed at clearing the infection. Neural signals are also generated to coordinate inflammation. However, in the asthmatic individual, immune response is altered and bronchial epithelial cells are damaged. A damaged bronchial epithelial lining is more susceptible to penetration by environmental irritants, which can directly stimulate sensory nerves leading to increased histamine release from mast cells and induction of smooth muscle cell contraction (i.e. bronchoconstriction) by released kinins [37]. A number of mediators are released by infected bronchial epithelial cells and play a role in recruiting major inflammatory cells, such as eosinophils, neutrophils and mast cells, though these cells may already be present in asthmatic airways. Eosinophils can be recruited to inflammatory sites by cytokines, most notably the T-helper type 2 (Th2) cell-derived interleukin (IL)-4, IL-5 and IL-13, and chemokines CCL5/RANTES and CCL11/eotaxin-1 [38], while neutrophils are most likely recruited by CXCL8/IL-8 or leukotriene B4[39]. In turn, mast cells can be activated by eosinophil-derived major basic protein to release a number of mediators [40]. Together, these major inflammatory cells release mediators that can cause airway hyperresponsiveness, airway remodeling (through the action of eosinophils, fibrogenic and growth factors), and airway limitation (bronchoconstriction, mucosal edema, hypersecretion), leading to characteristic asthma exacerbation symptoms (wheeze, dyspnea, cough, chest tightness).
Viral infection of bronchial epithelial cells leads to release of mediators, including cytokines, chemokines and growth factors. Recruited immune cells (eosinophils, neutrophils and mast cells) release a number of mediators as well. CCL = CC chemokine ligand; CXCL = CXC chemokine ligand; FGF-2 = fibroblast growth factor 2; GM-CSF = granulocyte macrophage colony-stimulating factor; LT = leukotriene; MBP = major basic protein; PAF = platelet-activating factor; PG = prostaglandin; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor.
Viral infection of bronchial epithelial cells leads to release of mediators, including cytokines, chemokines and growth factors. Recruited immune cells (eosinophils, neutrophils and mast cells) release a number of mediators as well. CCL = CC chemokine ligand; CXCL = CXC chemokine ligand; FGF-2 = fibroblast growth factor 2; GM-CSF = granulocyte macrophage colony-stimulating factor; LT = leukotriene; MBP = major basic protein; PAF = platelet-activating factor; PG = prostaglandin; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor.
Eosinophils are generally considered as major effector cells of asthma, and it has long been thought that the eosinophilic response to viral infection has a predominantly negative effect on human health, and that it is the major cause of inflammation responsible for tissue damage, bronchoconstriction and respiratory dysfunction [41]. However, several studies have shown that eosinophils may promote viral clearance and antiviral host defense. This concept, termed the ‘double-edged sword’, was first introduced by Smith [42] in 1994, with respect to the dual role of neutrophils in viral infection. Rosenberg and Domachowske [43,44,45] followed with their hypothesis that eosinophils may be recruited in part to promote primary antiviral host defense, possibly in situations where acquired immune response was less than effective [46]. More specifically, through their secretory mediators, eosinophils could reduce the infectivity of RSV for target epithelial cells in vitro. More recently, Phipps et al. [47] demonstrated accelerated clearance of RSV from murine lungs, and that full antiviral activity was dependent on intact Toll-like receptor signaling in eosinophils introduced exogenously.
In the past decade, a new hypothesis regarding asthma pathogenesis has materialized. Through numerous observations it has been noted that lower airway epithelium in asthmatics is structurally and functionally defective. More specifically, this concept incorporates the idea of persistent activation of the epithelium with signaling to the underyling structural cells and has been called the ‘epithelial-mesenchymal trophic unit’ (EMTU) [48]. This unit could provide a microenvironment conducive to allergic sensitization, lead to different types of inflammation and predispose the airways to exacerbations. Activation of the EMTU may also provide the impetus for tissue remodeling resulting in loss of airway reversibility, reduced lung function and increased resistance to treatment in adults. Together with environmental agents, such as air pollutants, tobacco smoke, drugs and other allergens, viruses can cause epithelial damage through the activation of the EMTU to amplify inflammatory and remodeling responses in the submucosa [49]. Structurally, the epithelium has defective tight junction formation associated with impaired barrier function [50], while functionally, the asthmatic epithelium was more sensitive to oxidant injury [51]. Furthermore, it exhibited profound impairment of virus-induced interferon-β mRNA expression and produced >2.5 times less interferon-β protein in response to respiratory viral infection, culminating in cytotoxic cell death and enhanced viral replication and shedding [52]. However, 2 other recent studies [53,54] were unable to reproduce these findings. Furthermore, a recent study of viral shedding after experimental HRV-16 inoculation of volunteers with and without asthma found no difference in viral shedding between these 2 groups [55]. In summary, it is still uncertain whether or not a defective epithelium may be at least partially responsible for the numerous chronic inflammatory and structural responses seen in chronic asthma.
Viral Infection and the Hygiene Hypothesis
Early childhood exposure to certain viruses may actually be protective against atopy and/or asthma, termed the ‘hygiene hypothesis’ [56]. This highly controversial idea was borne from early observations showing that the risk of developing allergy and/or asthma was inversely related to the number of children in the family [57]. However, this seems paradoxical, as it is also known that bronchiolitis and pneumonia in infancy lead to an increased risk of subsequent asthma. Whether infections have a protective effect or not may have to do with location, frequency, intensity and timing [58,59].
Other environmental factors influence the development of atopic sensitization, including early exposure to pets, increased use of antibiotics and an agricultural background [60]. In an agricultural setting, increased exposure to high levels of endotoxin has been associated with lower rates of allergy and an elevated number of interferon-producing cells in the blood [61,62], cells which are known for their potent anti-viral activity [63]. Taken one step further, Schaub et al. [64] demonstrated that maternal exposure to farming decreases the risk of allergic disease in offspring, possibly through the increased number and activation of T-regulatory cord blood cells associated with lower Th2 cytokine secretion and lymphocyte proliferation on innate exposure. Indeed, there is growing evidence, both in human and animal studies, that pre- and postnatal exposure to pathogens and allergens may provide a protective effect against allergy development. In addition, breastfeeding has been shown to protect against asthma and lower respiratory illness, especially RSV [65,66].
Viral Infection and Atopy
One paramount question that remains unanswered in determining the link between early childhood infection and asthma development is: do respiratory viral infections merely select those individuals who are predisposed to asthma, or are these infections able to alter lung development/immune response enough to actually cause asthma? A number of studies have shown that respiratory allergy may play a potentially synergistic role with viral infections in producing airway inflammation and subsequent asthma in childhood [20,58,65]. One recent cohort study by Kusel et al. [20] only found an association between viral infections during infancy and the subsequent development of persistent wheeze and asthma at the age of 5 years in children with atopic sensitization during the first 2 years of life. This association was also restricted to infections that spread to the lower respiratory tract and were intense enough to cause severe symptoms in the infants. Together, respiratory allergy and infection can cause airway dysfunction through several mechanisms, including viral infection damaging the barrier function of the airway epithelium, leading to enhanced absorption of aeroallergens [67], as well as the generation of various cytokines, chemokines, leukotrienes and molecules that may further upregulate cellular recruitment, cell activation and the continuing inflammatory response [37].
The Role of Eosinophilia in Asthma Development/Exacerbation
Eosinophilic inflammation is a cardinal feature of asthma, and increases in eosinophilic inflammatory markers have been shown to be good predictors of asthma exacerbations [68,69]. It has been demonstrated that suppression of eosinophilic inflammation with glucocorticosteroids is associated with an amelioration of symptoms and airway dysfunction [70]. A recent study by Fanat et al. [71] found that anti-IL-5 treatment reduced the eosinophilopoietic potential of airway smooth muscle cells, suggesting it may promote in situ eosinophilopoiesis in asthmatic lungs. Furthermore, Haldar et al. [72] and Nair et al. [73] demonstrated the ability of anti-IL-5 therapy in reducing eosinophil numbers while improving asthma control in refractory eosinophilic asthma.
Eosinophils as Effector Cells
Eosinophils are major effector cells of the allergic process. Activation of eosinophils leads to extracellular release of a number of granule proteins, such as eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), major basic protein and eosinophil peroxidase (EPO) (fig. 1) [74]. Among the many mediators released during eosinophil activation and degranulation, it is these eosinophil granule proteins that are the most strongly implicated in the pathophysiology of asthma [75]. Eosinophils also release a number of proinflammatory cytokines, chemokines and lipid mediators. In addition, eosinophils express a large number of cell surface markers, including adhesion and apoptotic signaling molecules, chemokine, complement and chemotactic factor receptors, and cytokine, immunoglobulin, prostaglandin, platelet-activating factor and leukotriene receptors [76,77,78,79,80].
Eosinophils have generally been regarded as terminal effector cells in allergic airway diseases; however, recent studies demonstrate their involvement in the initial stages of allergic disease development as well [81]. Murine studies suggest that eosinophils may actually drive T-cell responses as opposed to merely being driven by them. Eosinophils can act as antigen-presenting cells [82,83]; thus, at disease onset, they are able to induce activation and proliferation and/or cytokine production in T cells.
Viral Infection and Eosinophilia
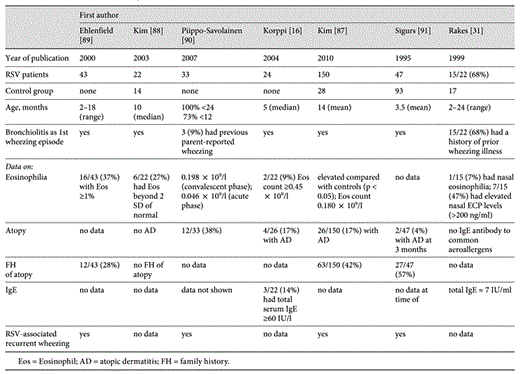
To further elucidate the connection between early childhood viral infection and asthma development, several studies involving RSV bronchiolitis have been carried out due to the clinical analogy, epidemiologic relationship and similar immunologic phenomena in the airways, such as specific IgE production [84], generation of chemokines [85] and adhesion molecules [86], as well as the eosinophil activation [87] which it shares with childhood asthma. A number of studies have demonstrated eosinophilia during/after RSV bronchiolitis [16,31,87,88,89,90,91] (table 1). However, the downfall of many RSV studies is the lack of premorbid eosinophil levels. Therefore, it cannot be explicitly stated that the observed eosinophilia was a result of RSV infection. Indeed, prenatal Th2/Th1 imbalance, allergic sensitization during pregnancy or some other reason may result in elevated eosinophil levels in infants. This was suggested in a study by Frischer et al. [92] of neonates born to atopic parents.
Though most viral infections are thought to be neutrophilic in nature, studies by our research group have shown that a subgroup of RSV bronchiolitis patients is eosinophil positive [88]. This subgroup also had higher levels of Th2 cytokines. Eosinophilia together with a skewed Th2 cytokine response strongly resembles the immunologic profile of childhood asthma. To further strengthen the relationship between RSV bronchiolitis and asthma, our research group recently undertook an RSV bronchiolitis study [87] investigating eosinophil degranulation after bronchiolitis and its association with recurrent wheezing. We found good correlations between the eosinophil degranulation marker, EDN, and recurrent wheezing episodes in a cohort of infants with a first episode of RSV bronchiolitis. Furthermore, EDN levels at 3 months proved to have predictive value for recurrent wheezing (positive predictive value 57%, negative predictive value 76%, sensitivity 72%, specificity 62%). Indeed, it appears that there is a subgroup of RSV bronchiolitis patients with marked eosinophilia that may go on to later develop asthma. This hypothesis may help elucidate the link between early childhood infections and asthma development.
Asthma Phenotypes
A number of techniques have recently been used to characterize the myriad of asthma phenotypes in hopes of better tailoring treatment to the individual. The classical IgE-associated allergic asthma phenotype (also termed ‘extrinsic’) starting in childhood is the most widely studied, as it is the most common in real-life and easily studied in the clinical laboratory. In allergic asthma, the cytokine profile is predominantly Th2 (i.e. IL-4, IL-5, IL-9 and IL-13). A recent genome microarray study by Woodruff et al. [93] found significant IL-13-mediated gene induction in asthma. One genetic biomarker in particular was associated with eosinophil accumulation in the airways, while another was identified as a potential mediator of corticosteroid-resistant asthma. Other studies, including the one by Martin et al. [94], have vetted other predictive markers, such as IgE and blood eosinophil levels, serum eosinophil degranulation protein levels, lung function and PC20[95]. These examples highlight the need for careful consideration of phenotypes, especially variable treatment responses, when treating asthma patients.
The less common ‘intrinsic’ asthma is of late onset, lacks circulating specific IgE, and no sensitivities to allergens can be identified [38]; despite this, airway eosinophilia and Th2 cells feature prominently [96]. Intrinsic asthma is less common (approx. 10%) than extrinsic asthma, is more common in females and tends to be more severe, requiring higher doses of corticosteroids [97]. However, clinically, intrinsic asthma shares a great deal with extrinsic asthma, including variable airflow obstruction and symptoms, and a good therapeutic response to corticosteroid therapy. It is well established that upper respiratory tract viral infections are the most common cause of exacerbations in intrinsic and extrinsic asthma [98].
In addition to eosinophilic inflammation, other cardinal features of most asthmatic phenotypes include airway hyperresponsiveness, excessive airway mucus production and airway remodeling [38].
Eosinophils and Asthma
Eosinophil increases in the tissues, body fluids (e.g., sputum, peripheral blood, serum, bronchoalveolar lavage) and bone marrow have been noted in asthma [88,99,100]. These elevated levels have correlated well with disease severity, leading to the hypothesis that eosinophils are the major effector cells of asthma. One way in which they act as major effector cells is through the release of granule proteins, which induce tissue damage and dysfunction [101], as well as the further propagation of airway inflammation. Elevated levels of ECP [102] and EDN [103] have been noted in asthmatics, with higher levels of ECP [104] and EDN [105] found during asthma exacerbation when compared with healthy patients and those with stable asthma. This would suggest that airway inflammation associated with asthma exacerbation is characterized not only by an increase in the number of eosinophils, but also by an increase in airway eosinophil degranulation [106].
Monitoring of Asthma with Eosinophil Markers
Though eosinophils are important in the pathophysiology of asthma, eosinophil degranulation and its associated products, such as EDN and ECP, may be even more important. It has been suggested that the secretory activity of eosinophils – a combination of the concentration of eosinophils and their propensity to release degranulation products – may be a key marker of disease activity and is more accurately measured by eosinophil degranulation products such as EDN and ECP [107]. Kim et al. [108] found significant differences in EDN and ECP levels in asthmatics during both acute and stable phases when compared with controls. Thus, EDN, along with ECP, may aid in the diagnosis of asthma. It has been suggested by several groups that EDN is more useful than ECP in evaluating disease severity [108,109,110]. This may partially be due to the recoverability of EDN, as ECP is a sticky and more highly charged protein [101].
In terms of clinical utility, EDN levels are a more accurate biomarker of the underlying pathophysiology of asthma (i.e. eosinophilic inflammation); consequently, they provide an objective measure of the secretory activity of eosinophils. In children too young to fully participate in lung function tests, EDN levels may be useful as an alternative measurement of eosinophilic inflammation, but larger studies should be carried out to determine the reproducibility and repeatability for this purpose.
Other Asthma Markers
Fractional exhaled concentration of nitric oxide (FeNO) has recently emerged as a biomarker to aid in the diagnosis, treatment and monitoring of asthma. Measurement of FeNO is relatively easy, and several devices are readily available and not too costly [111]. In a recently published study of children aged 5–18 years, FeNO compared favorably with sputum eosinophil percentage and better than spirometry for confirming asthma diagnosis [112]. However, obtaining FeNO was easier and less time consuming than obtaining induced sputum. One area of interest would be an investigation of FeNO levels during virus-induced asthma exacerbations versus those levels found in non-viral-induced (e.g., allergens) asthma exacerbations.
Knuffman et al. [113] recently found that an elevated baseline level of FeNO was predictive of a favorable response to inhaled corticosteroids. However, FeNO is limited in this area, as a substantial number of patients with lower FeNO may still respond to inhaled corticosteroid treatment [111].
A recent meta-analysis [114] of the study of Green et al. [115] and 2 others [116,117] bolsters the hypothesis that treatment based on sputum eosinophil counts is more efficacious than treatment based on clinical symptoms and other traditional objective measures of lung function. However, serial methacholine challenge tests or sputum eosinophil measurements are time consuming, labor intensive and expensive. As a proposed alternative guide to anti-inflammatory treatment for asthma, FeNO has demonstrated little to no benefit [111]. Furthermore, in studies of asthma control, induced sputum eosinophils have consistently performed as well or better than FeNO [118].
Conclusion
Viral infections are important causes of childhood respiratory disease. They can lead to long-term morbidity, such as recurrent wheezing, and evidence points toward a very strong link between early childhood infection and asthma development. However, the underlying pathophysiologic link between infection and chronic allergic disease is still not fully understood. With increased understanding and attention to these disease processes, clinicians will better be able to diagnose, treat and monitor asthma. Indeed, the focus must shift from not only treating symptoms, but also the underlying pathophysiology, to provide optimal care to the patient.