Abstract
Background: The Na+,Cl- coupled creatine transporter CreaT (SLC6A8) is expressed in a variety of tissues including the brain. Genetic defects of CreaT lead to mental retardation with seizures. The present study explored the regulation of CreaT by the ubiquitously expressed glycogen synthase kinase GSK3ß, which contributes to the regulation of neuroexcitation. GSK3ß is phosphorylated and thus inhibited by PKB/Akt. Moreover, GSK3ß is inhibited by the antidepressant lithium. The present study thus further tested for the effects of PKB/Akt and of lithium. Methods: CreaT was expressed in Xenopus laevis oocytes with or without wild-type GSK3ß or inactive K85RGSK3ß. CreaT and GSK3ß were further expressed without and with additional expression of wild type PKB/Akt. Creatine transport in those oocytes was quantified utilizing dual electrode voltage clamp. Results: Electrogenic creatine transport was observed in CreaT expressing oocytes but not in water-injected oocytes. In CreaT expressing oocytes, co-expression of GSK3ß but not of K85RGSK3ß, resulted in a significant decrease of creatine induced current. Kinetic analysis revealed that GSK3ß significantly decreased the maximal creatine transport rate. Exposure of CreaT and GSK3ß expressing oocytes for 24 hours to Lithium was followed by a significant increase of the creatine induced current. The effect of GSK3ß on CreaT was abolished by co-expression of PKB/Akt. Conclusion: GSK3ß down-regulates the creatine transporter CreaT, an effect reversed by treatment with the antidepressant Lithium and by co-expression of PKB/Akt.
Introduction
The creatine transporter CreaT (SLC6A8), a member of the superfamily of sodium and chloride coupled transporters for neurotransmitters [1,2,3] and organic osmolytes [4,5], is expressed in a variety of tissues including brain, retina, skeletal muscle, heart, and several epithelia [6,7,8,9]. Defects of the CreaT (SLC6A8) gene lead to mental retardation with seizures [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27].
Neuronal excitability [28] and cell volume [29] are regulated by the glycogen synthase kinase GSK3ß, a serine/threonine kinase inhibited by the antidepressant Lithium [30]. GSK3ß is phosphorylated and down-regulated by protein kinase B (PKB/Akt) [31].
The present study explored, whether CreaT activity is modified by GSK3ß. To this end, CreaT was expressed in Xenopus laevis oocytes without or with additional expression of wild type GSK3ß, inactive mutant K85RGSK3ß or wild-type GSK3ß together with wild type PKB/Akt. Creatine induced current determined by dual electrode voltage clamp was taken as measure of CreaT transport activity.
Materials and Methods
Ethical Statement
All experiments conform with the 'European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes' (Council of Europe No 123, Strasbourg 1985) and were conducted according to the German law for the welfare of animals and the surgical procedures on the adult Xenopus laevis frogs were reviewed and approved by the respective government authority of the state Baden-Württemberg (Regierungspräsidium) prior to the start of the study (Anzeige für Organentnahme nach §36).
Constructs
Constructs encoding bovine wild-type CreaT (SLC6A8) [3] (kindly provided by J Lingle), wild-type human GSK3ß [32], inactive mutant K85RGSK3ß [33], and wild-type PKB [34] were used for generation of cRNA as described previously [35,36,37,38].
Voltage clamp in Xenopus laevis oocytes
Xenopus laevis oocytes were prepared as previously described [37,38]. 15 ng cRNA encoding CreaT (SLC6A8) and 7.5 ng of cRNA encoding wild-type or inactive kinase were injected on the same day after preparation of the oocytes [36,39,40,41]. The oocytes were maintained at 17°C in ND96-A, a solution containing (in mM): 88.5 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, 2.5 NaOH, 5 HEPES, 5 Sodium pyruvate, Gentamycin (100 mg/l), Tetracycline (50 mg/l), Ciprofloxacin (1.6 mg/l), and Theophiline (90 mg/l). The pH was titrated to 7.4 by addition of NaOH [38,42]. Where indicated 1 mM Lithium chloride was added. The voltage clamp experiments were performed at room temperature 4 days after the first injection. Two-electrode voltage-clamp recordings were performed at a holding potential of -60 mV. The data were filtered at 10 Hz and recorded with a Digidata A/D-D/A converter (1322A Axon Instruments) [34,43]. The Clampex 9.2 software was used for data acquisition and analysis (Axon Instruments) [34,41,44]. The control superfusate (ND96-B) contained (in mM): 93.5 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 2.5 NaOH and 5 HEPES, pH 7.4. The flow rate of the superfusion was approx. 20 ml/min, and a complete exchange of the bath solution was reached within about 10 s [45].
Statistical analysis
Data are provided as means ± SEM, n represents the number of oocytes investigated. As different batches of oocytes may yield different results, comparisons were always made within a given oocyte batch. All voltage clamp experiments were repeated with at least 3 batches of oocytes; in all repetitions qualitatively similar data were obtained. Data were tested for significance using ANOVA or t-test, as appropriate. Results with p < 0.05 were considered statistically significant.
Results
In order to test whether the glycogen synthase kinase GSK3ß modifies the function of the creatine transporter CreaT (SLC6A8), cRNA encoding CreaT was injected into Xenopus laevis oocytes with or without additional injection of cRNA encoding GSK3ß. Creatine-induced current was determined by dual electrode voltage clamp and taken as measure of transport. As shown in Fig. 1, addition of creatine to the superfusate did not elicit an appreciable current in water-injected oocytes. Accordingly, the oocytes did not express significant endogenous electrogenic creatine transport. In CreaT expressing oocytes, however, the addition of creatine to the superfusate was followed by appearance of an inward current. The additional co-expression of wild-type GSK3ß was followed by a significant decrease of creatine-induced current in CreaT expressing Xenopus laevis oocytes.
Effect of wild-type GSK3ß or inactive mutant K85RGSK3ß on electrogenic creatine transport in CreaT (SLC6A8) expressing Xenopus laevis oocytes. A: Representative original tracings showing Icreat in Xenopus laevis oocytes injected with water (a), expressing CreaT alone (b) or expressing CreaT with additional co-expression of wild-type GSK3ß (c), or inactive K85RGSK3ß (d). B: Arithmetic means ± SEM (n = 10-16) of IcreatXenopus laevis oocytes injected with water (dotted bar) or expressing CreaT without (white bar) or with wild-type GSK3ß (black bar), or inactive K85RGSK3ß (grey bar) *** (p<0.001) indicates statistically significant difference from oocytes expressing CreaT alone.
Effect of wild-type GSK3ß or inactive mutant K85RGSK3ß on electrogenic creatine transport in CreaT (SLC6A8) expressing Xenopus laevis oocytes. A: Representative original tracings showing Icreat in Xenopus laevis oocytes injected with water (a), expressing CreaT alone (b) or expressing CreaT with additional co-expression of wild-type GSK3ß (c), or inactive K85RGSK3ß (d). B: Arithmetic means ± SEM (n = 10-16) of IcreatXenopus laevis oocytes injected with water (dotted bar) or expressing CreaT without (white bar) or with wild-type GSK3ß (black bar), or inactive K85RGSK3ß (grey bar) *** (p<0.001) indicates statistically significant difference from oocytes expressing CreaT alone.
In contrast to wild-type GSK3ß, the inactive K85RGSK3ß mutant did not significantly modify the creatine-induced current in CreaT expressing Xenopus oocytes (Fig. 1).
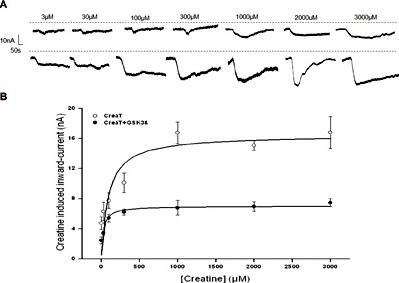
In order to test whether GSK3ß co-expression modifies the maximal creatine-induced current or the affinity of the carrier, the current induced by creatine concentrations ranging from 3 µM to 3 mM was determined in Xenopus laevis oocytes expressing CreaT without or with additional expression of wild-type GSK3ß. As illustrated in Fig. 2, the creatine-induced current was a function of the extracellular creatine concentration. Kinetic analysis revealed that the maximal creatine-induced current was significantly (p<0.001) higher in Xenopus laevis oocytes expressing CreaT alone (17.66 ± 1.97 nA, n = 6) than in Xenopus laevis oocytes expressing CreaT together with wild-type GSK3ß (6.88 ± 0.45 nA, n = 6). The concentration required for half-maximal creatine-induced current tended to be higher in Xenopus oocytes expressing CreaT alone (185.06 ± 96.50 µM, n = 6) than in Xenopus oocytes expressing CreaT together with wild-type GSK3ß (21.16 ± 6.62 µM, n = 6). Due to the large scatter of the calculated values, however, the difference did not reach statistical significance.
Electrogenic creatine transport as a function of creatine concentration in CreaT (SLC6A8)-expressing Xenopus laevis oocytes without or with additional expression of GSK3ß. A: Representative original tracings showing the current induced by increasing concentrations of creatine (from 3 µM to 3 mM) in Xenopus laevis oocytes expressing CreaT without (upper panel) or with (lower panel) additional co-expression of wild-type GSK3ß. B: Arithmetic means ± SEM (n = 6) of Icreat as a function of creatine concentration in Xenopus laevis oocytes expressing CreaT without (white circles), or with (black circles) additional co-expression of wild-type GSK3ß.
Electrogenic creatine transport as a function of creatine concentration in CreaT (SLC6A8)-expressing Xenopus laevis oocytes without or with additional expression of GSK3ß. A: Representative original tracings showing the current induced by increasing concentrations of creatine (from 3 µM to 3 mM) in Xenopus laevis oocytes expressing CreaT without (upper panel) or with (lower panel) additional co-expression of wild-type GSK3ß. B: Arithmetic means ± SEM (n = 6) of Icreat as a function of creatine concentration in Xenopus laevis oocytes expressing CreaT without (white circles), or with (black circles) additional co-expression of wild-type GSK3ß.
As GSK3ß could be inhibited by Lithium, additional experiments were performed in Xenopus oocytes expressing both CreaT and wild-type GSK3ß with or without prior exposure to 1 mM Lithium. As illustrated in Fig. 3, Lithium-treatment significantly enhanced the creatine-induced current in Xenopus oocytes expressing both CreaT and wild-type GSK3ß.
Effect of Lithium on electrogenic creatine transport in CreaT (SLC6A8) and GSK3ß expressing Xenopus laevis oocytes. A: Representative original tracings showing Icreat in Xenopus oocytes injected with water (a), expressing CreaT alone (b), or expressing CreaT with additional co-expression of wild-type GSK3ß without (c) or with (d, e) prior exposure to Lithium [1 mM] for 24 hours. B: Arithmetic means ± SEM (n = 9-16) of Icreat in Xenopus oocytes injected with water (dotted bar) or expressing CreaT alone (white bars), or expressing CreaT together with wild-type GSK3ß (black bars) either without (left bars) or with (right bars) prior exposure to 1mM Lithium for 24 hours. ***(p< 0.001) indicates statistically significant difference from Xenopus oocytes expressing CreaT alone, ### (p< 0.001) indicates statistically significant difference from the absence of Lithium.
Effect of Lithium on electrogenic creatine transport in CreaT (SLC6A8) and GSK3ß expressing Xenopus laevis oocytes. A: Representative original tracings showing Icreat in Xenopus oocytes injected with water (a), expressing CreaT alone (b), or expressing CreaT with additional co-expression of wild-type GSK3ß without (c) or with (d, e) prior exposure to Lithium [1 mM] for 24 hours. B: Arithmetic means ± SEM (n = 9-16) of Icreat in Xenopus oocytes injected with water (dotted bar) or expressing CreaT alone (white bars), or expressing CreaT together with wild-type GSK3ß (black bars) either without (left bars) or with (right bars) prior exposure to 1mM Lithium for 24 hours. ***(p< 0.001) indicates statistically significant difference from Xenopus oocytes expressing CreaT alone, ### (p< 0.001) indicates statistically significant difference from the absence of Lithium.
GSK3ß is phosphorylated and thus inactivated by protein kinase PKB/Akt. Additional experiments thus explored whether the effect of GSK3ß could be reversed by additional co-expression of PKB/Akt. As illustrated in Fig. 4, the co-expression of PKB/Akt significantly enhanced the creatine-induced current in Xenopus oocytes expressing both CreaT and wild-type GSK3ß.
Effect of additional co-expression of PKB on creatine transport in CreaT (SLC6A8) and GSK3ß expressing Xenopus laevis oocytes. A: Representative original tracings showing Icreat in Xenopus laevis oocytes injected with water (a), expressing CreaT alone (b) or with additional co-expression of wild-type GSK3ß (c), or wild-type GSK3ß and PKB/Akt (d). B: Arithmetic means ± SEM (n = 9-13) of Icreat in Xenopus laevis oocytes injected with water (dotted bar) or expressing CreaT without (white bar) or with wild-type GSK3ß (black bar), or GSK3ß +PKB/Akt (grey bar). ** (p<0.01) indicates statistically significant difference from oocytes expressing CreaT alone, ## (p<0.01) indicates statistically significant difference from oocytes expressing CreaT and GSK3ß.
Effect of additional co-expression of PKB on creatine transport in CreaT (SLC6A8) and GSK3ß expressing Xenopus laevis oocytes. A: Representative original tracings showing Icreat in Xenopus laevis oocytes injected with water (a), expressing CreaT alone (b) or with additional co-expression of wild-type GSK3ß (c), or wild-type GSK3ß and PKB/Akt (d). B: Arithmetic means ± SEM (n = 9-13) of Icreat in Xenopus laevis oocytes injected with water (dotted bar) or expressing CreaT without (white bar) or with wild-type GSK3ß (black bar), or GSK3ß +PKB/Akt (grey bar). ** (p<0.01) indicates statistically significant difference from oocytes expressing CreaT alone, ## (p<0.01) indicates statistically significant difference from oocytes expressing CreaT and GSK3ß.
Discussion
The present study discloses a novel regulator of the creatine transporter CreaT (SLC6A8), i.e. the glycogen synthase kinase GSK3ß. Co-expression of the wild-type GSK3ß but not of the inactive mutant K85RGSK3ß was followed by a significant decrease of creatine-induced inward current reflecting electrogenic creatine transport. GSK3ß thus contributes to the complex regulation of this widely expressed carrier.
The effect of GSK3ß was dissipated by the additional co-expression of protein kinase B which is known to phosphorylate and thus to negatively regulate GSK3ß [31]. Moreover, the effect of GSK3ß on CreaT was blunted by the antidepressant Lithium, a known inhibitor of GSK3ß [30].
The present observations did not address the molecular mechanisms involved in the down-regulation of CreaT activity by GSK3ß. In theory the kinase could be effective by directly phosphorylating the carrier-protein or by phosphorylating proteins involved in the regulation of trafficking or function of the carrier. CreaT has previously been shown to be regulated by JAK2 [46], JAK3 [45], AMPK [47,48], PKC [49], SPAK [39], OSR1 [39], PIKfyve [50], mTOR [51], SGK1 [52], SGK3 [52], klotho [38], and peroxisome proliferator-activated receptor-gamma co-activator-1alpha (PGC-1α) or beta (PGC-1β) [53].
Dual electrode voltage clamp in Xenopus oocytes is a powerful experimental approach to uncover interactions between signalling molecules and carriers. Observations made utilizing this experimental approach have frequently been confirmed by analysing gene targeted mice lacking or overexpressing the respective kinase or mutants [35,41,42,54,55,56,57,58,59]. Nevertheless, the present study does not allow safe predictions as to the functional significance of GSK3ß-sensitive CreaT activity. In the brain, CreaT accomplishes synaptic re-uptake of the neurotransmitter creatine and thus influences GABAergic and glutamatergic neurotransmission [47]. In theory, the observed effect of GSK3ß on CreaT could thus contribute to the known effect of the kinase on neuronal excitability [28] and on cell size [29]. Specifically, stimulation of GSK3ß phosphorylation and thus inhibition of GSK3ß has been shown to facilitate translocation of the Ca2+-sensitive and depolarization-induced transcription factor NFAT [28] and to increase of cell size [29].
In conclusion, GSK3ß down-regulates CreaT activity, an effect possibly influencing neuroexcitation and cell volume regulation.
Acknowledgements
The authors acknowledge the meticulous preparation of the manuscript by Lejla Subasic and technical support by Elfriede Faber. This study was supported by the Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of Tuebingen University .
Disclosure Statement
The authors of this manuscript state that they do not have any conflict of interests and nothing to disclose.




![Fig. 3. Effect of Lithium on electrogenic creatine transport in CreaT (SLC6A8) and GSK3ß expressing Xenopus laevis oocytes. A: Representative original tracings showing Icreat in Xenopus oocytes injected with water (a), expressing CreaT alone (b), or expressing CreaT with additional co-expression of wild-type GSK3ß without (c) or with (d, e) prior exposure to Lithium [1 mM] for 24 hours. B: Arithmetic means ± SEM (n = 9-16) of Icreat in Xenopus oocytes injected with water (dotted bar) or expressing CreaT alone (white bars), or expressing CreaT together with wild-type GSK3ß (black bars) either without (left bars) or with (right bars) prior exposure to 1mM Lithium for 24 hours. ***(p< 0.001) indicates statistically significant difference from Xenopus oocytes expressing CreaT alone, ### (p< 0.001) indicates statistically significant difference from the absence of Lithium.](https://karger.silverchair-cdn.com/karger/content_public/journal/cpb/40/5/10.1159_000453177/2/m_000453177_f03.jpeg?Expires=1716410878&Signature=CrKL1-9loQTJR61u0Hk09KGWRuLRCgUlWB0L7MOCerOADzw~xI5~ABY2votLedCrhvmW146tj~sNyimx73KgBsqYBrpm7kDB4OiLcWyYsJiSS~lB8UveXNyIdfhzdwenraxvH6atNOMRtZPGRSMmftImNCbJeUII6AbIjh5qfbjbWR5OzBbe7RLKPDEAn9WyVZSl8uVoptMXDVgPW6iMU97h3Gvb7GcOEtRu-vFF5V07XmE7KaEBn0-PpQHRMcOYscn1YIZSHfMvD~wAKU5zpag5Ii7hlZyTDyQ~0v1w40zBaBEju344zvOSb6iMkKMHdHrIM6oBjcqXA6ZJBr0OJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

