Abstract
Evolution has generated mammalian brains that vary by a factor of over 100,000 in mass. Despite such tremendous diversity, brain scaling in mammalian evolution has tacitly been considered a homogeneous phenomenon in terms of numbers of neurons, neuronal density, and the ratio between glial and neuronal cells, with brains of different sizes viewed as similarly scaled-up or scaled-down versions of a shared basic plan. According to this traditional view, larger brains would have more neurons, smaller neuronal densities (and, hence, larger neurons), and larger glia/neuron ratios than smaller brains. Larger brains would also have a cerebellum that maintains its relative size constant and a cerebral cortex that becomes relatively larger to the point that brain evolution is often equated with cerebral cortical expansion. Here I review our recent data on the numbers of neuronal and nonneuronal cells that compose the brains of 28 mammalian species belonging to 3 large clades (Eulipotyphla, Glires, and Primata, plus the related Scandentia) and show that, contrary to the traditional notion of shared brain scaling, both the cerebral cortex and the cerebellum scale in size as clade-specific functions of their numbers of neurons. As a consequence, neuronal density and the glia/neuron ratio do not scale universally with structure mass and, most importantly, mammalian brains of a similar size can hold very different numbers of neurons. Remarkably, the increased relative size of the cerebral cortex in larger brains does not reflect an increased relative concentration of neurons in the structure. Instead, the cerebral cortex and cerebellum appear to gain neurons coordinately across mammalian species. Brain scaling in evolution, hence, should no longer be equated with an increasing dominance of the cerebral cortex but rather with the concerted addition of neurons to both the cerebral cortex and the cerebellum. Strikingly, all brains appear to gain nonneuronal cells in a similar fashion, with relatively constant nonneuronal cell densities. As a result, while brain size can no longer be considered a proxy for the number of brain neurons across mammalian brains in general, it is actually a very good proxy for the number of nonneuronal cells in the brain. Together, these data point to developmental mechanisms that underlie evolutionary changes in brain size in mammals: while the rules that determine how neurons are added to the brain during development have been largely free to vary in mammalian evolution across clades, the rules that determine how other cells are added in development have been mostly constrained and to this day remain largely similar both across brain structures and across mammalian groups.
Introduction
Although the cellular composition of the brain is considered one of the major determinants of its computational capacities [Williams and Herrup, 1988], little was actually known until recently about how it scales with brain size, which varies by a factor of 100,000 across mammalian species [Count, 1947]. Does brain size reflect the number of brain neurons in the same manner across all mammalian species? That is, do brains of a same size always have similar numbers of neurons and larger brains thus necessarily more neurons than smaller ones?
The Previous View: ‘All Brains Scale the Same’
Comparative studies of mammalian brain anatomy with regard to brain scaling in evolution have largely been either volumetric analyses or comparisons of cell density across species small and large. The former studies have established that brain size is related to body size by a power law of exponents inferior to 1.0 [Martin, 1981; Fox and Wilczynski, 1986] such that brain size increases at a slower pace than body size and at different rates across mammalian orders [Marino, 1998]. Within the brain, the cerebral cortex increases faster in volume than do the remaining brain structures, gaining relative size in such a way that larger brains are more and more dominated by cortex but also at different rates across orders [Frahm et al., 1982]. The relative increase in the size of the cerebral cortex has come to be equated with brain evolution and is often offered as an explanation for our cognitive superiority compared to other species [Rakic, 2009]. In comparison, larger brains have isometrically larger cerebella, which accompany almost linearly the size of the cerebral cortex, and retain a stable relative size with increasing brain size so that larger brains have cerebella of the same relative volume. Depending on whether emphasis was placed on the absolute size or on the relative size of these structures as proxies for their absolute or relative numbers of neurons, these studies apparently supported either a tendency towards relative expansion of the role of the cerebral cortex in evolution [Clark et al., 2001] or the coordinated evolution of the roles of the cerebral cortex and cerebellum [Sultan, 2002] – both of which cannot be simultaneously true.
Comparisons of cell densities, in turn, were often made irrespectively of mammalian order and with a heavy bias on analyses of the cerebral cortex ever since Franz Nissl, based on visual inspection of the brains of various unrelated species, observed that neurons are distributed more sparsely in larger brains [Nissl, 1898]. Further studies soon supported his observation, showing that neuronal density declines in the cerebral cortex as a power function of increasing brain volume across unrelated species with a negative exponent of –0.32 [Tower and Elliott, 1952] and that the decrease in neuronal density with increasing brain size applies to a large group of species comprising from the smallest insectivores [Stolzenburg et al., 1989] to primates, dolphins, elephants, and whales [Tower, 1954; Garey and Leuba, 1986; Haug, 1987].
The decreased neuronal density in larger cerebral cortices is attributable to 2 factors: increased neuronal size (including the neuropil) and an increased relative number of interspersed glial cells. The glia/neuron ratio, which expresses this relative number of glial cells distributed among the neurons [Friede, 1954], apparently increases with brain size when compared across mammalian species as diverse as cat, mole, opossum, baboon, dolphin, whale, elephant, and man [Hawkins and Olszewski, 1957; Haug, 1987]. Interestingly, the increased glia/neuron ratio is not accompanied by any major variation in glial density, which has been reported either to vary widely but independently of brain size [Haug, 1987] or to remain stable [Tower and Young, 1973; Stolzenburg et al., 1989] across mammalian species of increasing brain size. Because of these supposedly universal neuronal and glial scaling rules, glia are widely said to be the most numerous cell type in the brain [Doetsch, 2003; Nishiyama et al., 2005] and to be 10–50× more numerous than neurons in humans [Kandel et al., 2000]. Evidence for this assertion, however, is scant.
We now know that these presumed scaling rules actually do not apply universally to mammals, as reviewed next. In retrospect, the original views on brain scaling in evolution were confounded by comparisons across few species, irrespectively of mammalian order, and by unreliable or not directly comparable cell density measurements. For instance, neuronal densities in human cerebral cortex have been estimated at as low as 8,750/mm3 [Tower and Elliott, 1952] and as high as 48,100/mm3 [Shariff, 1953], with intermediate estimates of 24,500/mm3 [Von Economo, 1926], 25,000/mm3 [Haug, 1987], and 41,300/mm3 [Pope, 1978]. Moreover, multiplying such density estimates by the volume of the structure to determine total numbers of neurons, as attempted by Haug [1987] and Stevens [2001], necessarily yields numbers that are heavily biased by structure volume and thus likely incorrect. Using modern stereology to determine total numbers of cells in the brain and its main structures in a volume-independent manner to then infer average neuronal density and glia/neuron ratios would be a daunting task that would either require parcelling the brain into hundreds of structures of homogeneous cell density or very sophisticated sampling schemes. As a consequence of all of these shortcomings, there were few direct data on how total numbers of neuronal and glial cells compare across species or on what scaling rules apply to how different mammalian brains vary in size as they gain neuronal and nonneuronal cells – until recently.
A Nonstereological Approach to Determining Total Numbers of Cells: The Isotropic Fractionator
The isotropic fractionator is a method developed recently in our lab which allows the nonstereological determination of the absolute number of neuronal and nonneuronal cells in different brain regions [Herculano-Houzel and Lent, 2005]. It consists of transforming highly anisotropic brain structures into homogeneous, isotropic suspensions of fixed, free cell nuclei which can then be counted and identified immunocytochemically as neuronal or nonneuronal. The method can be applied either to the brain as a whole or to its dissected parts, such as the cerebral cortex or cerebellum, whose respective numbers of cells can next be added up in order to obtain a whole-brain estimate. Estimates of total neuronal and nonneuronal numbers in any brain structure can be obtained in 24 h, with a coefficient of variation below 10%. Since the estimates obtained are independent of brain mass or volume, they can be used in comparative studies of variation in brain size among species and in studies of phylogenesis, development, adult neurogenesis, and pathology.
We have so far used the isotropic fractionator to compare the numbers of cells that compose the entire adult brain [divided into the cerebral cortex (grey and white matter combined), cerebellum (grey and white matter and deep nuclei combined), and the rest of the brain (RoB), excluding the olfactory bulb] of 28 mammalian species that can be grouped into 3 large clades (fig. 1): 10 Glires (9 rodents and 1 lagomorph) [Herculano-Houzel et al., 2006], 12 primates (including humans) [Herculano-Houzel et al., 2007; Azevedo et al., 2009; Gabi et al., 2010] plus the closely related tree shrew (order Scandentia; the tree shrew is, however, not included in the primate data set for quantification and will be analyzed separately), and 5 Eulipotyphla (insectivores) [Sarko et al., 2009]. The following is a review of the cellular scaling rules found to apply within each of these clades or across them.
Phylogenetic relationships between the 10 Glires, 12 primate, 5 insectivore, and 1 scandentia species examined [data based on Blanga-Kanfi et al., 2009; Purvis, 1995; Murphy et al., 2004].
Phylogenetic relationships between the 10 Glires, 12 primate, 5 insectivore, and 1 scandentia species examined [data based on Blanga-Kanfi et al., 2009; Purvis, 1995; Murphy et al., 2004].
Different Body-Brain Relationships
Across the 28 species examined, body mass varies almost 10,000-fold, from about 8 g in the smoky shrew to over 70 kg in humans, while brain mass varies by over 8,000×, accompanied by a smaller 3,200× variation in the total number of brain neurons. Variations in these parameters are, as a whole, correlated with one another; larger species tend to have larger brains and larger numbers of neurons in the brain. There is not, however, a single universal relationship across these parameters that applies to all 3 clades. Glires and Eulipotyphla seem to share a body-brain relationship (Glires, MBR = 0.037 MBD0.712; Eulipotyphla, MBR = 0.044 MBD0.727) which is, however, clearly distinct from the body-brain relationship that applies to primates (MBR = 0.029 MBD0.922; fig. 2a) in that, for similarly sized bodies, primates always have larger brains than insectivores and Glires. Most importantly, relationships between body mass and the number of brain neurons are distinct across the 3 groups (Glires, NBR = 15.17 × 106 × MBD0.451; Eulipotyphla, NBR = 8.19 × 106 × MBD0.717; Primata, NBR = 5.94 × 106 × MBD0.801) in that, for a same body size, primates have even larger numbers of neurons compared to Glires than expected from their brain mass, and insectivores have smaller bodies than Glires with a similar number of brain neurons (fig. 2b).
Scaling of brain structure mass and number of brain neurons as a function of body mass. Each point represents the average brain mass (a) and number of neurons in the brain (b) of a species belonging to the mammalian orders indicated to the right. Power functions, listed in table 1, are not plotted so as not to obscure the data points.
Scaling of brain structure mass and number of brain neurons as a function of body mass. Each point represents the average brain mass (a) and number of neurons in the brain (b) of a species belonging to the mammalian orders indicated to the right. Power functions, listed in table 1, are not plotted so as not to obscure the data points.
The heterogeneous scaling relationships between body mass and the number of brain neurons can be accounted for by the different scaling relationships between brain mass and the number of brain neurons across the 3 clades. Variations in brain mass can be described as power functions of the number of brain neurons within each clade (table 1). Brain mass increases rapidly with variation in the number of brain neurons raised to the power of 1.5 in Glires, but it increases approximately linearly with variation in the total number of brain neurons in primates or insectivores (table 1). As a result, a brain with a 10× larger number of neurons becomes 32× larger if it belongs to a Glire but only about 10× larger in a primate or insectivore.
The different scaling relationships that apply to brain size, brain number of neurons, and body size among themselves and across orders can be appreciated by ranking the 28 species in the data set according to each of these parameters (fig. 3). While insectivores have the smallest bodies and brains (fig. 3a, b), they outrank several larger Glires in number of neurons (fig. 3c). Similarly, while Glires and primates are largely overlapping in range of body sizes (fig. 3a), it is the primates that have the largest numbers of neurons in the sample (fig. 3c). Brain size, therefore, is not a universal proxy for the number of brain neurons across mammals, which also bears no strict relation to body size.
Different species ranking based on body mass, brain mass, and number of brain neurons. Species listed in figure 1 [within each plot, Eulipotyphla (a), Glires (b), and Primata and Scandentia (c)] are shown ranked from left to right by increasing body mass (a), brain mass (b), or number of brain neurons (c). Notice that the spacing between species is not drawn to scale.
Different species ranking based on body mass, brain mass, and number of brain neurons. Species listed in figure 1 [within each plot, Eulipotyphla (a), Glires (b), and Primata and Scandentia (c)] are shown ranked from left to right by increasing body mass (a), brain mass (b), or number of brain neurons (c). Notice that the spacing between species is not drawn to scale.
Different Brains Gain Neurons in Different Ways
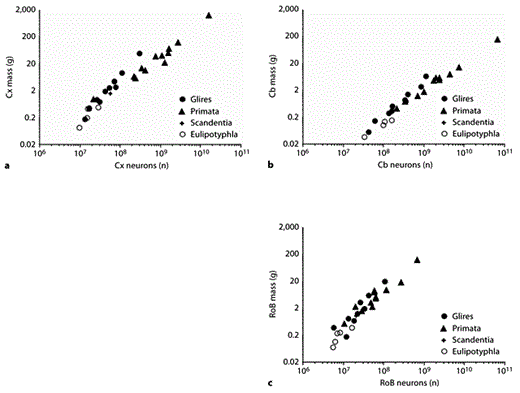
Similarly sized cerebral cortices can contain remarkably different numbers of neurons in Glires and primates. For instance, while the agouti cortex, at 17.7 g, holds 795 million neurons, the slightly smaller owl monkey cortex, at 15.7 g and 1.5 billion neurons, contains almost twice as many [Herculano-Houzel et al., 2006, 2007]. The larger the mass of the cerebral cortex, the larger the discrepancy in numbers of neurons between Glires and primates (fig. 4a). This is because the structure is found to scale in mass as different functions of its number of neurons across the 2 clades: as a power function of exponent 1.7 in Glires and as a power function of exponent 1.1 in primates (table 1) that is equally well fitted as a linear function [Gabi et al., 2010]. The Glire cerebral cortex, therefore, gains neurons in a very volume-costly manner, while the primate cortex gains neurons more economically in terms of resulting structure volume. The insectivore cerebral cortex, in turn, overlaps with Glires in its neuronal scaling and shares a similarly large allometric exponent of 1.6 (table 1; fig. 4a), increasing in size very rapidly as it gains neurons.
Clade- and structure-specific scaling of brain structure mass as a function of numbers of neurons. Each point represents the average mass and number of neurons in the cerebral cortex (a), cerebellum (b), or RoB (c) of a species belonging to the mammalian orders indicated on the right. Power functions, listed in table 1, are not plotted so as not to obscure the data points. Cx = Cerebral cortex; Cb = cerebellum.
Clade- and structure-specific scaling of brain structure mass as a function of numbers of neurons. Each point represents the average mass and number of neurons in the cerebral cortex (a), cerebellum (b), or RoB (c) of a species belonging to the mammalian orders indicated on the right. Power functions, listed in table 1, are not plotted so as not to obscure the data points. Cx = Cerebral cortex; Cb = cerebellum.
The cerebellum, like the cerebral cortex, gains mass faster than it gains neurons in Glires, with an allometric exponent of 1.3 (table 1; fig. 4b). In primates and insectivores, in contrast, cerebellar mass scales linearly with the number of cerebellar neurons (table 1; fig. 4b). As a result, primate and insectivore cerebella are found to contain many more neurons than do Glire cerebella of a similar mass. For instance, the bonnet monkey cerebellum, at 5.7 g, contains 2 billion neurons; this is almost twice as many as the capybara cerebellum at 6.6 g and 1.2 billion neurons [Herculano-Houzel et al., 2006, 2007]. The eastern mole cerebellum, at 0.15 g, contains 158 million neurons; this is over twice as many neurons as the hamster cerebellum at 0.14 g and 61 million neurons has [Herculano-Houzel et al., 2006; Sarko et al., 2009].
Interestingly, the relationship between the mass of the remaining brain structures that compose the RoB and its number of neurons does not appear as clearly separated across the 3 clades, with a much larger overlap among the data points (fig. 4c) and exponents (table 1; the primate exponent increases to 1.4 after correction for phylogenetic relatedness in the data set) [Gabi et al., 2010]. This raises the interesting possibility that the scaling rules for the RoB, in contrast to the cerebral cortex and cerebellum, are shared across Glires, primates, and insectivores.
Despite the different scaling rules that apply to the cerebral cortex and cerebellum across the 3 clades, we find that both structures gain neurons coordinately across the 28 species (fig. 5), as originally reported for the initial data set of 19 species [Herculano-Houzel, 2010], at an average rate of 4.2 neurons in the cerebellum to every neuron in the cerebral cortex. Such a coordinated addition of neurons to the 2 structures is compatible with the modern view of the integrated function of the cerebral cortex and cerebellum and supports the notion that the 2 structures are subject to similar selective pressures and evolve concertedly [Whiting and Barton, 2003; Ramnani et al., 2006; Balsters et al., 2010]. The coordinated scaling of their numbers of neurons is masked by their different mass scaling relationships, given that the cerebral cortex increases in mass as it gains neurons with a higher exponent than the cerebellum, as described above, so that its relative mass increases in larger primate and Glire brains.
Coordinated scaling of the numbers of neurons in the cerebellum and cerebral cortex of mammals. Each point represents the average number of neurons in the cerebellum (y-axis) and cerebral cortex (x-axis) of a species belonging to the mammalian orders indicated on the right. The scaling is best described as linear, with a slope of 4.2 for the entire data set (95% CI 4.0–4.4) or slopes of 3.9 (Glires, 95% CI 2.6–5.2), 4.3 (primates, 95% CI 4.0–4.6), or 7.2 (Eulipotyphla, 95% CI 0.2–14.2). Cx = Cerebral cortex; Cb = cerebellum.
Coordinated scaling of the numbers of neurons in the cerebellum and cerebral cortex of mammals. Each point represents the average number of neurons in the cerebellum (y-axis) and cerebral cortex (x-axis) of a species belonging to the mammalian orders indicated on the right. The scaling is best described as linear, with a slope of 4.2 for the entire data set (95% CI 4.0–4.4) or slopes of 3.9 (Glires, 95% CI 2.6–5.2), 4.3 (primates, 95% CI 4.0–4.6), or 7.2 (Eulipotyphla, 95% CI 0.2–14.2). Cx = Cerebral cortex; Cb = cerebellum.
Indeed, the distribution of mass in the brain does not reflect the distribution of neurons across the cerebral cortex and cerebellum. In none of the 3 clades is the relative number of neurons in either structure (calculated as a percentage of all brain neurons) correlated with the relative mass of the structure (Spearman’s correlation, p > 0.05; table 1). This indicates that a relatively larger cerebral cortex does not hold relatively more neurons in larger brains. Rather, the cerebral cortex typically contains around 20% of all brain neurons, while the cerebellum holds 70–80% of all brain neurons [Herculano-Houzel, 2009] regardless of brain size and inclusive of the human brain [Azevedo et al., 2009]. In primates and Glires, however, larger brains do possess relatively fewer neurons in the RoB; the percentage of brain neurons situated in the RoB decreases with brain mass raised to the power of –0.174 in Glires (p = 0.0415) and –0.283 in primates (p = 0.0003; table 1).
Different Species and Brain Structures Gain Nonneuronal Cells in the Same Manner
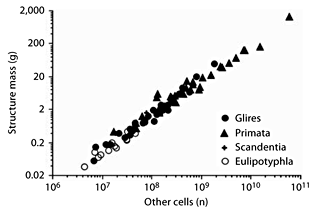
In contrast to the clade-specific rules that apply to how the cerebral cortex and cerebellum scale in size as they gain neurons, the rules that govern the addition of nonneuronal (other) cells to the brain appear to be shared not only across clades but also across brain structures. As shown in figure 6, the cerebral cortex, cerebellum, and RoB scale in size as similar, overlapping power functions of their respective numbers of other cells raised to exponents of 0.9–1.1 (or as linear functions of their numbers of other cells; table 1). As a result of the approximately linear relationship between brain structure mass and the number of other cells, we find that the 3 structures share a similar range of densities of other cells which do not correlate significantly with variations in structure mass (fig. 7; table 1).
Shared scaling of brain structure mass in the combined data set as a function of numbers of other cells. Each point represents the average mass and number of other cells in the cerebral cortex, cerebellum, or RoB of each mammalian species in the data set. Power functions are not plotted so as not to obscure the data points, which are largely overlapping across structures.
Shared scaling of brain structure mass in the combined data set as a function of numbers of other cells. Each point represents the average mass and number of other cells in the cerebral cortex, cerebellum, or RoB of each mammalian species in the data set. Power functions are not plotted so as not to obscure the data points, which are largely overlapping across structures.
Other-cell density is largely overlapping across structures. Each point represents the average structure mass and other-cell density (number of other cells/milligram) in the cerebral cortex, cerebellum, or RoB of each species. O = Number of other cells.
Other-cell density is largely overlapping across structures. Each point represents the average structure mass and other-cell density (number of other cells/milligram) in the cerebral cortex, cerebellum, or RoB of each species. O = Number of other cells.
Average Neuronal Size Scales in a Structure- and Clade-Specific Manner
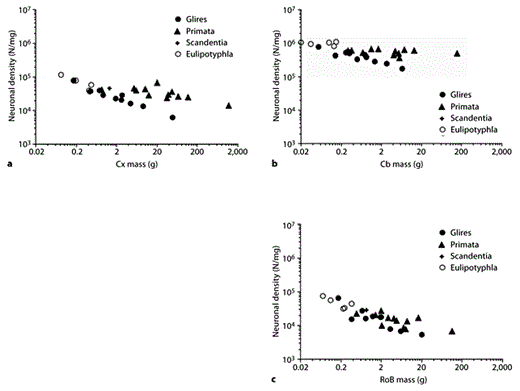
In contrast to the relatively stable other-cell density, neuronal cell densities are highly variable across species and structures. In Glires and Eulipotyphla, the cerebral cortex increases in size with an accompanying steep decrease in neuronal density (fig. 8a), which varies with cortical mass raised to the power of –0.424 or –0.569, respectively (table 1). In primates, there is only a slight decrease in neuronal density in larger cortices, with an exponent of –0.168 (table 1). On the other hand, the cerebellum scales in size with no significant change in neuronal density in Eulipotyphla and primates and with a more modest decrease in neuronal density in Glires (fig. 8b; table 1). Likewise, only in Glires does an increase in RoB mass correlate with a decrease in neuronal density, which varies with RoB mass raised to the power of –0.467 (fig. 8c). Consistent with the possibility that the RoB neuronal scaling rules are shared across the 3 clades, however, the distributions of neuronal densities in this structure are fairly overlapping across the 3 clades.
Neuronal cell densities scale differently across structures and orders. Each point represents the average structure mass and neuronal cell density (number of neurons/milligram) in the cerebral cortex (a), cerebellum (b), or RoB (c) of each species. N = Number of neurons; Cx = cerebral cortex; Cb = cerebellum.
Neuronal cell densities scale differently across structures and orders. Each point represents the average structure mass and neuronal cell density (number of neurons/milligram) in the cerebral cortex (a), cerebellum (b), or RoB (c) of each species. N = Number of neurons; Cx = cerebral cortex; Cb = cerebellum.
In light of the relatively constant other-cell densities across structures, decreases in neuronal cell density can be interpreted as an indication of increased average neuronal size (which includes the cell soma as well as all of the dendritic and axonal arbors and the pericellular space; similarly, the average nonneuronal cell size includes all of their arbors and the pericellular space). Thus, it can be inferred that the cerebral cortex scales in mass as a function of a larger number of neurons of rapidly increasing average size in Glires and Eulipotyphla and slowly increasing size in primates, if at all; the cerebellum scales in size as a function of increasing numbers of neurons of relatively constant size in Eulipotyphla and primates but of increasing average mass in Glires, and the RoB scales with more neurons of increasing average size in Glires but of not significantly different size in primates and Eulipotyphla.
Glia/Neuron Ratio Scales Homogeneously with Neuronal Density
Nonneuronal cells include all glial cell types, ependymal cells, and endothelial cells. Because the latter are estimated to amount to at most 5% of all brain cells, given the small relative volume of brain vasculature [Lauwers et al., 2008], and because the relative number of ependymal cells is most likely very small, the nonneuronal/neuronal cell ratio serves as an upper limit of the glia/neuron ratio and provides a reasonable approximation of its actual value. For simplicity, the nonneuronal/neuronal ratio in our sample will hereinafter be referred to as the glia/neuron ratio.
Contrary to what is commonly assumed in the literature [Marino, 2006], we find no general trend of larger brains (or brain structures) having larger glia/neuron ratios. Because of the different combinations of shared nonneuronal scaling rules and clade- and structure-specific neuronal scaling rules, the glia/neuron ratio is found to increase together with structure size only in the cerebral cortex, cerebellum, and RoB of Glires and in the cerebral cortex of Eulipotyphla (fig. 9a). Remarkably, however, we find that the variation in the glia/neuron ratio accompanies decreasing neuronal density in a manner that is overlapping across all structures and species (although it does not reach significance within the cerebellum; fig. 9b; table 1) and therefore appears to obey a shared scaling rule that, like the addition of nonneuronal cells to brain tissue, is clade and structure nonspecific. Moreover, analysis of published data for the cerebral cortex shows that the same inverse relationship between the glia/neuron ratio and neuronal density is found across species as diverse as cetaceans, primates, elephants, and insectivores (fig. 9c). Because of the inverse relationship between neuronal density and average neuronal size, this finding suggests that the glia/neuron ratio is directly related to the average neuronal size: the larger the average neuronal size in a structure, whatever the species, the larger the glia/neuron ratio in the structure.
Glia/neuron ratio scales differently across structures and orders with structure mass but scales homogeneously with neuronal density. Each point represents the average glia/neuron ratio and structure mass (top) or neuronal density (center and bottom) in the cerebral cortex (grey), cerebellum (black), or RoB (black) of a species. c Data for cerebral cortex only [from Haug, 1987; Stolzenburg et al., 1989]. Notice that in contrast to the scattered distribution across species and structures in (a), data points are aligned across species and structures in (b) and across species of different orders in (c). N = Number of neurons; Cx = cerebral cortex; Cb = cerebellum.
Glia/neuron ratio scales differently across structures and orders with structure mass but scales homogeneously with neuronal density. Each point represents the average glia/neuron ratio and structure mass (top) or neuronal density (center and bottom) in the cerebral cortex (grey), cerebellum (black), or RoB (black) of a species. c Data for cerebral cortex only [from Haug, 1987; Stolzenburg et al., 1989]. Notice that in contrast to the scattered distribution across species and structures in (a), data points are aligned across species and structures in (b) and across species of different orders in (c). N = Number of neurons; Cx = cerebral cortex; Cb = cerebellum.
We have proposed [Herculano-Houzel et al., 2006] that the universal scaling both of brain structure mass with the number of other (glial) cells and of the glia/neuron ratio with neuronal density results from the same mechanism: the regulation of gliogenesis (which, we suggest, generates cells of relative invariant average size across species) by the size of the neuronal parenchyma that is invaded by glial precursors in early postnatal development [Sauvageot and Stiles, 2002; Bandeira et al., 2009]. Glial precursor proliferation is density-dependent and ceases once a steady-state glial density has been achieved, most likely by cell-cell contact inhibition [Zhang and Miller, 1996]. Given the relatively invariant nonneuronal densities we and others [Tower and Young, 1973; Haug, 1987] observed both across brain structures and species, we suggest that continued gliogenesis until confluency is reached in a formerly purely neuronal tissue is a candidate mechanism by which glial and neuronal cell numbers are related in that the mass of a given brain tissue is directly related to its number of glial cells while the glia/neuron ratio depends simply on the average size of the neurons. Considering that the average size of glial cells does not scale while the average neuronal size may or may not scale, those structures with large neurons will, by this mechanism, have large glia/neuron ratios while those with small neurons will accordingly have small glia/neuron ratios (fig. 10).
Glia/neuron ratio scales with average neuronal size. The scheme depicts 2 identical volumes of brain tissue which have similar glial cell densities (dark grey) and different neuronal cell densities owing to the different average neuronal cell sizes (light grey). Because glial cells are proposed to occupy the tissue homogeneously and to not vary significantly in average cell mass together with brain size, changes in average neuronal size either across structures or across species will result in corresponding changes in the glia/neuron ratio. G = Glia; N = neuron.
Glia/neuron ratio scales with average neuronal size. The scheme depicts 2 identical volumes of brain tissue which have similar glial cell densities (dark grey) and different neuronal cell densities owing to the different average neuronal cell sizes (light grey). Because glial cells are proposed to occupy the tissue homogeneously and to not vary significantly in average cell mass together with brain size, changes in average neuronal size either across structures or across species will result in corresponding changes in the glia/neuron ratio. G = Glia; N = neuron.
A Tree Shrew-Like Common Ancestor of Glires and Primates?
A final point concerns the position of the tree shrew along the distribution of brain size and number of neurons. The tree shrew is currently classified in the order Scandentia, which, together with Rodentia, Lagomorpha, and Primata (as well as the Dermoptera, not analyzed here) composes the superorder Euarchontoglires [Murphy et al., 2004]. The cellular composition of the tree shrew brain can be predicted very well by the primate neuronal scaling rules, deviating on average by only 12.5% of the predicted numbers of neurons in the different brain structures, by 28.3% from the values predicted by the Glires neuronal scaling rules, and by a larger 42.0% from the predictions for Eulipotyphla. The good alignment with primates suggests that orders Scandentia and Primata, although considered sister clades, nevertheless share the same neuronal scaling rules – just like the sister orders Rodentia and Lagomorpha.
Intriguingly, in the distributions of brain structure mass against numbers of neurons, the tree shrew is positioned approximately at the intersection between Glires and primates (fig. 4, 8). Although this placing might be meaningless since the 2 distributions are bound to intersect at some point, it raises the interesting possibility that the tree shrew brain is similar to the brain that once belonged to the original ancestral species that gave rise to the extant Euarchontoglires, branching in the 2 directions that evolved into Glires and Primata, with their clade-specific neuronal scaling rules.
Conclusions
By undertaking a systematic comparison of the quantitative neuronal and nonneuronal composition of different mammalian brains, our long-term goal, through establishing what rules are shared among mammalian brains (and thus might reflect characteristics inherited and retained from a common ancestor) and what rules differ across orders of mammals (and thus reflect phylogenetic variance across groups), is to uncover the brain developmental mechanisms that have either been conserved or subjected to changes in evolution. So far, by comparing Euarchontoglires (Glires and Primata) and 1 group of Laurasiatheria (Eulipotyphla), we have found that there are, indeed, some scaling rules that are shared among clades (and even among brain structures); these are the rules that apply to how brain structures scale as they gain nonneuronal (presumably glial) cells, with relatively invariant nonneuronal cell densities and a glia/neuron ratio that is inversely related to the average size of the neurons in the structure. Brain size, as a consequence, is a very good proxy for the number of nonneuronal (glial) cells in the brain.
The rules that apply to the scaling of brain structures as they gain neurons, in turn, are both clade and structure specific. Brain size, therefore, is not a good proxy for the number of neurons in a brain in cross-order comparisons and is particularly misleading when making comparisons of cognitive abilities related to numbers of neurons: an elephant brain, although larger, does not necessarily have more neurons than a human brain and indeed might have significantly fewer [Herculano-Houzel, 2009]. The impact of the clade-specific neuronal scaling rules can already be appreciated in comparisons between similar-sized rodents and primates: because of the higher neuronal densities in primate brains compared to rodent brains of equivalent size (about 40,000 neurons/mg in the cortex of Aotus against 12,000/mg in the agouti, e.g.) while maintaining similar nonneuronal cell densities (which indicates that the average neuronal size is smaller in primates than in rodents), primate brains contain more neurons than do rodent brains of equivalent size. The larger number of neurons per unit volume presumably endows primates with a larger computational capacity than rodent brains of equivalent size. This type of evolutionary change allowed primate brains to accumulate large numbers of neurons without becoming prohibitively large: a macaque brain of 6.4 billion neurons built with the neuronal scaling rules that apply to Glires would, for example, weigh 575 g instead of its actual 87 g. These findings suggest that the divergence of primate evolution away from the common ancestor with rodents involved mechanisms that favored either a reduction in average neuronal cell size or the ability to add neurons to brains without making them larger, for instance through circuitry changes that favored local connectivity [Herculano-Houzel et al., 2010].
The evolutionary implications of the clade- and structure-specific neuronal scaling rules with putatively universal glial scaling rules are intriguing; in mammalian brain evolution, it appears that neurons (supposedly of each of the various neuronal cell types, although that remains to be examined) have been largely free to vary in size across structures and species, while glial cells, however variable in their morphology [Walz, 2000; Barres, 2008], do not quite vary in size across species or even across structures, maintaining very similar properties among mammals [Picker et al., 1981; Mishima and Hirase, 2010] and even in amphibia [Kuffler et al., 1966]. We do predict a small degree of variation in glial cell size in correlation with neuronal cell size [Mota and Herculano-Houzel, pers. observ.], and this is in agreement with recent findings in the literature comparing mouse and human astrocytes [Oberheim et al., 2009]. Since most of these nonneuronal cells can be expected to be glial cells, this finding is compatible with the idea that astrocytes are homogeneously distributed within the gray matter and occupy the parenchyma dividing it into polyhedric territories of similar volume [Bushong et al., 2002; Ogata and Kosaka, 2002; Nedergaard et al., 2003; Halassa et al., 2007], as well as with the observation that gray matter astrocytes in the cerebral cortex and hippocampus are morphologically and electrophysiologically homogeneous [Mishima and Hirase, 2010]. Together with these observations, our findings indicate that glial cell evolution is severely constrained, which in turn suggests that glial cells as a whole perform such a fundamental job that their structure and function can hardly be messed with. This is in agreement with the intricate functional and metabolic interactions between neurons and glia that have been found to apply to human and rat brains alike [Sibson et al., 1998; Magistretti et al., 1999; Shen et al., 1999]. Indeed, the shared scaling of brain size with numbers of glial cells suggests that the glial characteristics that apply today to extant brains were present in the common ancestor of the current 28 species over 90 million years ago [Murphy et al., 2004]. Extending our analysis to other mammalian clades should reveal whether the shared glial scaling rules observed here apply to mammals as a whole and thus reflect scaling rules that have been maintained since the last common ancestor that gave rise to mammals about 230 million years ago [Murphy et al., 2004].
Acknowledgments
Thanks to Jon Kaas and Roberto Lent for their continued support and encouragement, and to our many collaborators for their involvement in acquiring the data on which this review is based. This work is supported by grants from CNPq, FAPERJ, MCT/CNPq/FAPESP, and the James S. McDonnell Foundation.



![Fig. 1. Phylogenetic relationships between the 10 Glires, 12 primate, 5 insectivore, and 1 scandentia species examined [data based on Blanga-Kanfi et al., 2009; Purvis, 1995; Murphy et al., 2004].](https://karger.silverchair-cdn.com/karger/content_public/journal/bbe/78/1/10.1159_000327318/2/m_000327318_f01.gif?Expires=1716310044&Signature=sxQjwSh6~f6eCJgbJbua81N8KVvKWTJTRR4uy1T-rCTPF14wimZl6OrwsOKbcMFFIFe8lZx-Vox22iDIfD3vEXs6iyxzzWEBtdHmD-RrLAtjFn8fXO~QkpdMLRKdMx0idm47V47dLT7Jb~HEOy3mA~0TPTCOcj3dsYfisuqUl5ii752s7pJ89nXx~NAkXR~ZSE4aeGhlsKIGjQA-TlBdm-l4V9fGyCmM3puRbwsLCwFfYzNoY56AN3Yo41fSLvnk5dR44ufuSQwOPMC4j750us2LPItAVD0woD~yDR7zskXoWNQKVpNCo4SiKY0Ub~8R7ha0jf9iTstAzck-sRB2jg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 3. Different species ranking based on body mass, brain mass, and number of brain neurons. Species listed in figure 1 [within each plot, Eulipotyphla (a), Glires (b), and Primata and Scandentia (c)] are shown ranked from left to right by increasing body mass (a), brain mass (b), or number of brain neurons (c). Notice that the spacing between species is not drawn to scale.](https://karger.silverchair-cdn.com/karger/content_public/journal/bbe/78/1/10.1159_000327318/2/m_000327318_f03.gif?Expires=1716310044&Signature=IsZ0549NX72us2nznoJBd5RHtOlSjgW15l~HuxugEEUg4QKGD5e9AwUcpMqrgNpcHe8WdpIitsY0dc7slUKGAG8IBOrKWSkpGvfrw~goRRbbg6AclxF1olGAJ3zKmPMWy5wxZBv5K7Jw3Ibir-W0oxCJh2EtrCfFIx6OoAHL9Nith0IH76gliVJ6c7nXr-ZjmZW4CljpV2pcyUMs6ylTtGCQzBQ2BXySqG4i0y3Yx0LT6ytTRMBpEhLfZr7oNDUsYrnnFJ29P8oWZb078cWbOyQ0J2b-5M4EV6qjaxB0-BraRGcmmBZ2BendUyUU-V1MxjLBI~EuDfeU1D-lRgnqRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 9. Glia/neuron ratio scales differently across structures and orders with structure mass but scales homogeneously with neuronal density. Each point represents the average glia/neuron ratio and structure mass (top) or neuronal density (center and bottom) in the cerebral cortex (grey), cerebellum (black), or RoB (black) of a species. c Data for cerebral cortex only [from Haug, 1987; Stolzenburg et al., 1989]. Notice that in contrast to the scattered distribution across species and structures in (a), data points are aligned across species and structures in (b) and across species of different orders in (c). N = Number of neurons; Cx = cerebral cortex; Cb = cerebellum.](https://karger.silverchair-cdn.com/karger/content_public/journal/bbe/78/1/10.1159_000327318/2/m_000327318_f09.gif?Expires=1716310044&Signature=0r~Cxq7ga8ERM7QH2GAmnqUB2ljunqLDA4C-W6IzQtBIcqbLKMAorToBvMVU6tzaA4dxQBp702qd-i1Dnd1SjxCFg8Dhq0-HmL5Je4045ZHs2~kv3cP3kkbwyS6QRynG8eWfZF1iIP2JZUn64cQqD50zyrx0HS7dxo1gqSSvm4dAclh8LqsxhcN1NSi1U8R8Ea0KDJ2hNCURva9-ag3D6pL0QtN81zdBV3-FQKhszHNPP1zcHzTgRel5bnRA~rTsOqBYVq~4AJA1~ltwJFXpmP~IkIZZ-655~qEgYH4jbtg-pWdAPZq91oO1M4kNTgb6KCWrg7zlVyK4WINsGWrNtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
