Abstract
Molecular cytopathology is a rapidly evolving field of cytopathology that provides biological information about the response to personalised therapy and about the prognosis of neoplasms diagnosed on cytological samples. Biomarkers such as circulating tumour cells and circulating tumour DNA are increasingly being evaluated in blood and in other body fluids. Such liquid biopsies are non-invasive, repeatable, and feasible also in patients with severe comorbidities. However, liquid biopsy may be challenging due to a low concentration of biomarkers. In such cases, biomarkers can be detected with highly sensitive molecular techniques, which in turn should be validated and integrated in a complex algorithm that includes tissue-based molecular assessments. The aim of this review is to provide the cytopathologist with practical information that is relevant to daily practice, particularly regarding the emerging role of circulating tumour cells in the field of predictive molecular pathology.
Introduction
Molecular cytopathology is a rapidly evolving field of cytopathology based on cytology and “omics” technologies [1]. The recent advent of targeted treatment for many advanced solid tumours led to the widespread implementation of biomarker testing to select cancer patients potentially sensitive to these treatments [2, 3]. Tissue specimens are now considered the gold standard for molecular analysis. Core-needle biopsy and fine-needle aspiration (FNA) procedures are used to sample superficial and deep-seated lesions [4‒8]. FNA has several advantages over core-needle biopsy: (1) FNA can sample a larger area of the lesion; (2) FNA samples tumour cells that are less contaminated by stromal tissue, and (3) because FNA enables on-site adequacy assessments, the preliminary diagnosis can be immediately conveyed to clinical care providers [9]. However, the amount of tumour tissue sampled by FNA may not be sufficient for molecular testing, whereas the number of biomarkers to test in such solid tumours as non-small cell lung cancer (NSCLC) and colorectal cancer (CRC) is steadily increasing. Furthermore, treatment may alter the mutational status of tumours, which implies the need for serial samplings [10, 11]. However, re-biopsy after initial treatment is not always feasible in patients with comorbidity [11]. With the introduction of second-line targeted treatment with the EGFR tyrosine kinase inhibitor osimertinib in T790M-positive NSCLC patients, blood became an easily accessible source of biological material because, for the first time, a patient’s mutational status could be defined and dynamically monitored [12].
In the field of predictive molecular pathology, the analysis of biological components in blood and other body fluids is referred to as “liquid biopsy” [11, 13‒15]. From a clinical point of view, liquid biopsy is a non-invasive repeatable procedure that can be performed also in patients with severe comorbidities [13‒15]. Circulating tumour cells (CTCs) and circulating tumour DNA can be detected in whole blood, plasma, and serum [15‒17]. However, given the very low concentration of CTCs, highly sensitive techniques are required for their analysis, and these in turn should be validated and integrated in a complex algorithm that includes tissue-based molecular assessments (Fig. 1) [18‒20]. Consequently, liquid biopsy-based techniques represented a breakthrough in this setting [11, 13].
Schematic representation of CTC morphological evaluation and molecular analysis. (A) Cytopathologists play an important role in the morphological identification of CTCs from other non-neoplastic cells, with classical microscopic evaluation. CTC analysis allows information to be obtained regarding gene expression profiling assessment (B) and mutational analysis (C – circle plot for an exemplificative exome analysis) and protein expression (D), in order to obtain relevant information in predictive and prognostic settings.
Schematic representation of CTC morphological evaluation and molecular analysis. (A) Cytopathologists play an important role in the morphological identification of CTCs from other non-neoplastic cells, with classical microscopic evaluation. CTC analysis allows information to be obtained regarding gene expression profiling assessment (B) and mutational analysis (C – circle plot for an exemplificative exome analysis) and protein expression (D), in order to obtain relevant information in predictive and prognostic settings.
The aim of this review is to give the cytopathologist practical information regarding the liquid biopsy technique that is relevant to daily practice, and to illustrate the emerging role of CTCs in the field of predictive molecular pathology (Fig. 2).
2D and 3D culture applications. a CTCs isolated from blood samples were cultured in monolayer (2D) and prepared as a cell block. b Serial sections derived from the cell block were used for phenotypic (TTF-1, CK7, ALK, PD-L1) and molecular characterisation, identifying an EGFR exon 19 deletion (p.E746_A750delELREA). c 3D cultures prepared starting from isolated CTCs with EGFR exon 19 deletion were tested for IC50 assay using three EGFR tyrosine kinase inhibitors (gefitinib, erlotinib, and afatinib).
2D and 3D culture applications. a CTCs isolated from blood samples were cultured in monolayer (2D) and prepared as a cell block. b Serial sections derived from the cell block were used for phenotypic (TTF-1, CK7, ALK, PD-L1) and molecular characterisation, identifying an EGFR exon 19 deletion (p.E746_A750delELREA). c 3D cultures prepared starting from isolated CTCs with EGFR exon 19 deletion were tested for IC50 assay using three EGFR tyrosine kinase inhibitors (gefitinib, erlotinib, and afatinib).
Circulating Tumour Cells
From the earliest phases of tumour development, neoplastic cells, including CTCs, are actively shed into the bloodstream through the epithelial-to-mesenchymal transition (EMT) [21, 22]. The expression of EMT markers is associated with enhanced CTC invasion and migration as well as enhanced resistance to apoptosis and necrosis [23]. These cells can remain dormant for several years in specific “niches” (e.g., bone marrow) before giving rise to overt metastases [18, 24]. CTCs with an intermediate epithelial-mesenchymal phenotype, which is characterised by low expression of the epithelial cell adhesion molecule (EpCAM), and high expression of tyrosine-protein kinase, Met, are very aggressive and able to metastasise [18, 25‒28]. EpCAM is a protein exclusively expressed by epithelia and neoplasms of epithelial origin and are therefore used to select CTCs [25]. CTCs can also derive from neoplastic microemboli [29, 30]. The latter are composed of at least two tumour cells and, occasionally, by normal blood cells [19]. The formation of microemboli results from the increased adhesive capacity of CTCs, and improves the metastatic potential of these cells. Notably, CTCs elude the immune system through many immune checkpoint inhibitor systems such as programmed cell death protein 1 and programmed death-ligand 1 [31, 32].
The generally low concentration of CTCs in peripheral blood (usually 1–10 cells per 10 mL) and their half-life makes it difficult to distinguish CTCs from normal blood cells [19]. One strategy with which to distinguish between the two cell types is to analyse markers expressed by tumour cells (e.g., cytokeratin and EpCAM) but not by normal blood cells [18, 19]. However, false negative results can derive from the downregulation of epithelial markers that occurs during the EMT [18, 33]. The novel marker actin bundling protein plastin 3 is not downregulated during the EMT of CTCs and is not expressed in blood cells, and can thus overcome this problem [18, 34]. Other important issues remain to be addressed, namely false positive results due to artefacts and to the presence of CTCs in benign diseases (e.g., Crohn disease and diverticulosis) [18, 35].
Studies conducted in mouse models show that CTCs can serve as biomarkers for early solid tumours. For example, in a mouse model of pancreatic tumour, cancer cells were found in the bloodstream before any primary tumour was detectable [36, 37]. Moreover, particular molecular signatures can also predict the site of CTC metastases. For instance, in mouse xenograft experiments, EpCAM-negative CTCs in brain metastatic breast cancer were found to be characterised by the expression of selected markers (HER2+/EGFR+/HPSE+/NOTCH1+) that render these cells prone to generate brain metastases [38].
The analysis of CTCs can help to shed light on tumour cell dissemination and help to improve the management of metastatic disease [39]. In fact, DTCs (disseminated tumour cells) were included in the 2010 edition of the AJCC TNM (tumour-node-metastasis) cancer staging manual as cM0 (i+) classification [19]. In addition, targeting the dormant DTCs resident in niches like bone marrow can reduce the probability of metastases [24]. Although the clinical significance of CTCs is still debated [19], growing evidence indicates that the presence of CTCs in the bloodstream of cancer patients is an important prognostic factor and is predictive of the response to therapy [19]. The measurement of CTCs has not been included in clinical guidelines because their clinical utility is unclear, and they have yet to be validated in a prospective randomised clinical trial [19]. CTCs play a relevant role in small cell lung cancer (SCLC). In particular, CTCs were detectable in about 70–95% of patients with SCLC [40, 41]. The identification of CTCs may play an important role in the early detection of SCLC [40]. In the experience by Ilie et al. [42], the authors showed that all patients with the identification of CTCs developed a pulmonary nodule during the follow-up and before CT identification. A high level of CTCs in SCLC patients correlates with the worst prognosis [40]. In a study by Hou et al. [43], a high number of CTCs (> 300) was associated with a significant reduction of survival compared to patients with a low number of CTCs detected (< 2). Another important role of CTCs in these patients was represented by the possibility of a molecular characterisation of CTCs that can provide relevant clinical information [40]. Pore et al. [44] demonstrated the possibility that there was a relationship between the number of CTCs and overall survival rate, correlated to the presence of cancer stem cell (CSC) markers and mesenchymal markers in tumour biopsy samples.
CTC Selection
Various techniques have been devised to identify and isolate CTCs [18, 19, 45, 46]. The US Food and Drug Administration (FDA) approved the Cell-Search assay (Janssen Diagnostic, Raritan, NJ, USA) only for the enumeration of CTCs in breast and prostate cancer and CRC [33, 46, 47]. This assay is a protein expression-based technology by which CTCs are captured by immunomagnetic beads thanks to the expression of EpCAM on their cell surface [19, 20, 48, 49]. The advantage of this method is that EpCAM is not expressed on the surface of non-neoplastic circulating cells, while the principal disadvantage is the possibility of low EpCAM expression during the EMT. Other markers are being evaluated to address this problem [18, 19, 49‒53].
Other techniques are available to detect and isolate CTCs although they do not have FDA approval [18, 19, 54‒58]. For example, being larger and less prone to deform than hematopoietic cells, CTCs can be isolated by devices based on cell filtration and centrifugation [18, 19, 59]. In addition, functional assays, like the EPISPOT assay, are based on the detection of specific proteins secreted during in vitro culture of CTCs [18‒20, 60]. Besides not having FDA approval, the use of these assays in the era of multimarker assessment is much debated [18, 19]. The DEPArrayTM system (Silicon Biosystems, Bologna, Italy) is a technology based on a semiautomated system that allows the isolation of CTCs, with the capacity to generate a non-uniform electric field with the ability to develop an attractive force on polarisable elements (such as cells) that are suspended in a liquid [61, 62]. This principle is called dielectrophoresis (DEP) and allows fluorescently labelled CTCs to be isolated using a chip in which different microelectrodes create electric cages for the CTCs [61, 62]. Important advantages of this technique are that it allows individual cells to be trapped, manipulated, and recovered, with the activation and deactivation of the microelectrodes; it allows the identification of multiple cells and cell types in a sample; it does not create particular damage on cells due to the low voltage adopted [61, 62]. In a study by Paolillo et al. [63], the authors adopted the DEPArrayTM system in breast cancer liquid biopsy samples and the analysis of ESR1 (oestrogen receptor 1) mutation was correctly performed on all single cells isolated.
CTCs and Conventional Cytology
As a general rule, cytomorphological features can be used to increase the specificity of CTCs provided the integrity of the cells is well preserved [20, 64]. The isolation by size of epithelial tumour cells (ISET) technique complements standard cytopathology well [65]. The ISET method, based on the larger size of CTCs versus hematopoietic cells, has the advantage of highly sensitive enrichment while preserving cell integrity [65]. In a study by Hofman et al. [66], 10 mL of peripheral blood from 208 lung cancer patients were collected in buffered EDTA before surgical resection, and processed by the ISET technique. Using a modified May-Grünwald-Giemsa method to stain CTCs, they identified the microscopic features of malignancy in 102 of these patients. The agreement within a panel of 10 cytopathologists was excellent. In fact, in all 102 cases, at least 5/10 cytopathologists agreed on the classification of CTCs in three categories (CTCs with malignant features, with uncertain malignant features, and with benign features) using the following cytological criteria: irregularity and size of nucleus, anisonucleosis, nuclear hyperchromatism, nucleocytoplasmic ratio, size and number of nucleoli, and the presence of tridimensional sheets. In addition, using a semiquantitative analysis, the cytopathologists defined three groups: group 1 with less than 10 CTCs, group 2 with between 10 and 100 CTCs, and group 3 with more than 100 CTCs. Notably, the morphological features of circulating non-hematologic cells did not distinguish among the different histological subtypes. The presence of a preoperative high level of circulating non-hematologic cells was associated with a worse outcome for resectable lung cancer patients [66].
CTCs can also be visualised with such cytological methods as cytospin, ThinPrep, and cell block. Both the two principal cytological staining procedures, Romanowski and Papanicolaou, are performed on slides to visualise CTCs. Immunocytochemistry can also be used to detect diagnostic biomarkers [67].
Ex vivo Culture of CTCs and Chemosensitivity Assays
The choice of the most effective chemotherapy protocol after the eradication of the primary tumour mass is based on TNM staging and on the results of clinical trials. The identification of CTCs can help to improve personalised medicine for cancer patients. In fact, CTCs can be used to perform sensitivity assays to test the efficacy of such treatments in each patient [68, 69]. Moreover, CTCs detach from the primary mass and generate metastases, thereby overcoming the molecular heterogeneity of the tumour mass [68‒70].
CTCs can be used in predictive in vitro tests in individual patients. Indeed, the treatment response rate obtained with the CTC model was found to be closely associated with the response observed in the patient in vivo immediately after surgical removal of the tumour, and in metastatic patients [71‒73]. Therefore, CTCs can also be used to evaluate the response to treatment during the different phases of the disease, and treatment can be adjusted according to the evolution of the disease. In this context, Rüdiger et al. [72] demonstrated that the evaluation of chemosensitivity of CTCs and white cells comprising the circulating epithelial tumour cells (CETCs) in vitro is associated with the success of therapy in vivo and that it provides early information about the response to therapy.
In a recent ground-breaking study, in vitro drug sensitivity assays were performed in breast cancer patients with metastatic luminal oestrogen-positive breast cancers using ex vivo CTCs [74]. The authors generated at least one cell culture from 6 of 36 patients off therapy or progressing on treatment, but no cell culture lines from 9 patients responding to treatment [74]. Besides demonstrating that CTCs in culture shared most of the characteristics of CTCs directly isolated from blood, they also identified additional features. In particular, they found acquired mutations in the ESR1 and phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA) genes on CTCs in culture that were not present in the primary tumours [74]. Thanks to genotyping assessment, it is possible to evaluate the response to a given treatment based on the stratification of patients according to the mutations found [74].
CTCs extracted and expanded from CRC patients are a useful model for drug sensitivity testing in the framework of personalised medicine [75]. Grillet et al. [75] obtained three different CTC cell lines starting from four chemotherapy-naïve patients with metastatic CRC (stage IV). They were not able to obtain any CTC line from patients with lower stage (stage I/II, 0/9; stage III, 0/6) or chemotherapy-treated CRC patients (stage IV, 0/13; stage I/II, 0/3). They expanded cells and performed assays in less than 1 month [75], which is a critical point when CTC drug assays are performed to decide treatment. They found that the different cells lines responded differently to treatment with combinations of fluorouracil and irinotecan, and multikinase inhibitor regorafenib and vemurafenib. Once again, this is an important point since the results of in vitro tests can guide the oncologist’s choice regarding the best chemotherapeutic cocktail also in the attempt to avoid adverse side effects [75].
Drug sensitivity assays seem to be very promising in the field of breast cancer where it is often necessary to perform several biopsies to monitor the evolution of the malignancy, particularly in terms of the status of the oestrogen receptor and HER2 (human epidermal growth factor receptor 2) [74]. CTCs isolated from blood samples are useful in this context because the procedure is non-invasive and the cells can be used for genetic evaluation and drug sensitivity assays [76]. Notably, CTCs are currently being evaluated in clinical trials of breast cancer patients in the attempt to predict the response of patients to treatment with HER-targeted therapy [76‒79].
Chemosensitivity Assays Using Blood Samples
In 2014, Hughes et al. [80] reported a method to test the response to treatment in which chemotherapy compounds are added to the tube containing the blood sample, and then, after a well-defined time lapse, the CTCs present in the blood sample are counted. They found that response to treatment with docetaxel, doxorubicin, and mitoxantrone differed among blood samples obtained from 7 cancer patients (3 breast cancer, 2 prostate, 1 renal, and 1 colon). This test is rapid and simple and can be applied to different CTC isolation platforms. However, as acknowledged by the authors, a lower number of CTCs recovered after treatment may not always accurately reflect therapeutic efficacy [80]. Many clinical trials are now monitoring the CTC count during treatment of various cohorts of patients to verify whether CTC count is a valid predictive tool of response or relapse [78, 81]. In addition, testing CTC sensitivity to compounds in their natural medium (blood) could be another variable to use in the context of CTC testing in predictive medicine.
Three-Dimensional Cell Culture Models
In recent decades, the development of three-dimensional (3D) cell cultures has given an impulse to cancer research. In fact, the cells in the body work by interacting with the surrounding cells and with the extracellular matrix [82‒84]. Therefore, 3D models of cell cultures can be used to study proliferation, differentiation, apoptosis, migration, and invasion [85, 86]. Even more important is their use in studies of cytotoxicity and sensitivity to treatment since cells in 3D cultures are much more resistant to chemotherapy and radiotherapy versus traditional monolayer cultures (2D) [83, 85, 87‒89]. This explains why compounds that pass the first screening on cell culture in vitro are subsequently discarded due to their inefficacy on 3D cell models including CTC and animal models.
Three 3D cell culture models have been generated so far: (i) 3D spheroids [90, 91]; (ii) cultures in which natural and synthetic hydrogels and extracellular matrices serve as platforms, [92, 93] and (iii) cultures on solid scaffolds [94]. These models are applied in various settings, such as the differentiation of stem cells, the generation of organoids, studies of the biology of cancer cells, in multicellular models of cocultures, and finally, in generating organs on chips for drug discovery and toxicity studies [90, 92, 93].
Application of CTCs in 3D Cultures
3D cell cultures can be generated starting from tissues or cell lines, and a particularly promising application, although in its infancy, is in the field of CTCs. Obviously, such an application involves the use of particular expedients such as eliminating blood cells and cellular contaminants [74]. In fact, CTCs derived from breast cancer can proliferate as tumorspheres [74]. Although the tumorsphere model does not completely recapitulate the 3D architecture and biology of the tumour, it is useful for studies of stem cell differentiation [95]. In addition, CTCs obtained from the blood of breast cancer patients constitute an excellent tool with which to study drug susceptibility in relation to the unique genetic context of each individual tumour in the desirable perspective of personalised therapy [74].
It is difficult to obtain viable CTCs and many cells die during the purification procedure [45]. In their 2014 report, Yu et al. [74] used the CTC-iCHIP technology to purify and expand CTCs obtained from patients with oestrogen receptor-positive metastatic breast cancer. This result shows that such studies can be performed in patients who, being metastatic, cannot be biopsied [74].
The ability of CTCs to grow as 3D spheroids may directly depend on their tendency to remain viable in the bloodstream despite not being adhered to the extracellular matrix [74]. Bichsel et al. [96] showed that a microfluidic chip could be used as a culture chamber in 3D on the hydrogel matrix for prostate CTCs. Indeed, the captured cells do not detach from the support, and consequently are not lost, so that the CTCs can be expanded in a clonal and 3D fashion within the same support [96, 97]. The spheroids thus obtained can be used as models since they are sufficiently similar to the tumours from which they derive (expression of surface markers, response to drugs, cellular heterogeneity, stem properties) [98‒100], and so provide information about disease progression [96]. Moreover, these cells are clonogenic in vitro and can reform the tumour when injected in vivo [96, 101‒103]; these characteristics are almost certainly due to the maintenance of some stem-like properties [104, 105].
CTCs act as precursors of metastases, but their role in this process has only recently been clarified, mainly because CTCs are rare and it is often difficult to obtain an adequate number [106]. In fact, previously, CTCs were mostly isolated and amplified from animal models or from patients in advanced stages of disease [38, 74, 107]. However, a recent study showed that CTCs of early-stage lung cancer can be captured directly using a microfluidic chip by adding tumour-associated fibroblasts and the extracellular matrix in coculture to the 3D scaffold [106]. Thus, the optimal cellular microenvironment, where rare CTCs can be amplified and used for subsequent studies of invasion, the formation of spheroids, and gene sequencing, is recreated [106].
Another possible application of CTCs in 3D cultures in studies of the metastatic process was reported in a recent review [108]. Namely, it appears that the interaction between CTCs and the endothelial cells of vessels is modulated by mechanical factors such as blood flow [109, 110]. In addition, it has been seen that this interaction also depends on the physiological state of endothelial cells [111, 112]. These interactions can be studied, at least in principle, using appropriate 3D models that rebuild the interaction between cells within a microfluidic device [108].
Gao et al. [113] confirmed that organoids can be generated from CTCs obtained from patients with metastatic prostate cancer. They also showed that the organoid cell line can be long-term propagated. Thus, the results of molecular and genomic characterisation studies recapitulate the characteristics of the primary tumour [113]. Even more interesting is the possibility of genetically manipulating the organoid to study the response to treatment [113, 114]. In fact, gene transfer and gene targeting can be performed in liver and pancreas organoids using viral vectors (retrovirus, lentivirus, and adenovirus) and the CRISPR/Cas system, respectively [115].
Pizon et al. [116] recently reported that tumorspheres can be obtained starting from breast cancer CTCs. They selected a particular subpopulation of CTCs with stem cell characteristics using a specific culture medium and, intriguingly, showed that the number of tumorspheres obtained depends on the chemotherapeutic regimen to which the patient is subjected [116]. Thus, the CSC fraction of a CTC population can be monitored. The novelty of the study by Pizon et al. [116] is the use of the 3D growth of CTC to enrich the aggressive and probably metastases-generating CSC population [51, 116, 117]. This experimental method for the enrichment of circulating CSCs is well established in cultured cell lines and cells obtained by disrupting the primary tumour.
Li et al. [118], used CTCs from patients suffering from hepatocellular carcinoma to obtain the cellular subpopulations based on the different levels of pERK and pAKT to monitor the response to sorafenib in relation to the levels of phosphoproteins. They used a 3D model of spheroids [118, 119] to track the sensitivity and progression-free survival in all patients. This is another example of personalised therapy.
Perspectives on the Use of CTCs in 3D Cell Cultures
Given the widespread use of CTCs and liquid biopsy in recent years, mainly as a consequence of their non-invasive nature, it is feasible that their applications will increase in the fields of personalised medicine and the biology of metastases [120]. A series of applications in 3D cultures that best mimic the characteristics of the primary starting tumour have already been reported [82, 84‒86]. It has also been reported that CTCs grow for a longer time in 3D conditions than in 2D conditions [121].
A pioneering application of CTCs was reported by Mishra et al. [122] in 2015 in lung cancer. They used a 4D model (3D with liquid flow) of acellular rat lung, in which cells of interest were left to colonise the matrix, then examined the characteristics of nodules that appeared. They found that cells in 4D culture are more aggressive and release the CTCs responsible for the metastasis [122].
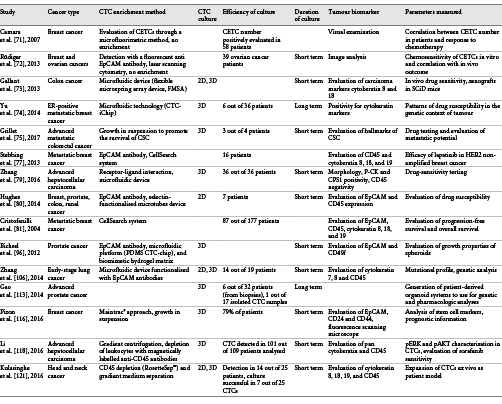
In conclusion, 3D cultures more closely resemble animal models than do 2D cultures, and can be used to study the complex interactions that occur within the body [123]. Their applications in basic and applied research are numerous and include drug discovery, cell physiology, tissue engineering, pharmacology, cancer research, and gene expression [123] (Table 1). Specifically, it seems that the amplification of rare CTCs is more efficient with the 3D cell culture models than with 2D models. In addition, they can also be used to study metastases and the processes of malignancy because growth experiments can be performed without anchorage to the substrate. Lastly, the response to treatment can be studied using CTCs in 3D models, thereby improving personalised medicine.
Acknowledgements
The authors thank Jean Ann Gilder (Scientific Communication srl., Naples, Italy) for writing assistance.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
Additional information
Dr. Malapelle and Dr. Troncone contributed equally to this article.