Abstract
Purpose: To report the anatomical and functional outcomes of Argus II retinal prosthesis implantation in Korean patients. Methods: We included 5 consecutive patients with end-stage retinitis pigmentosa (RP) who underwent Argus II retinal prosthesis implantation and were followed for at least 12 months. The transcorneal electrical evoked response was utilized for patient selection. We used intraoperative optical coherence tomography (OCT) for optimal placement of the array and provided specialized vision rehabilitation training. A morphological evaluation using SD-OCT and a functional evaluation using computer-based visual function tests, a letter-reading ability test, and the Functional Low-Vision Observer Rated Assessment (FLORA) were conducted. Results: Postoperatively, the array was completely apposed to the retinal surface in all eyes, except for one eye which had a preexisting macular concavity. Fibrosis-like tissues of ≥50-μm thickness developed at the interface in 2 eyes. All of the patients showed improvement in computer-based visual function tests and could read ETDRS letters at a distance of 50 cm. Three patients could read Korean words. FLORA was improved in all patients, mainly in tasks of visual mobility, daily activities, and social interactions. Conclusions: Along with good anatomical outcomes and specialized rehabilitation practices, recipients of the Argus II implant showed profound improvements in functional vision and mobility.
The Argus II retinal prosthesis system (Second Sight Medical Products, Inc., Sylmar, CA, USA) is approved for use in blind patients with outer retinal degeneration. Our team at the Asan Medical Center was the first in South Korea to implant retinal prosthesis in patients with end-stage retinitis pigmentosa (RP). The Korean Ministry of Food and Drug Safety officially approved the device in April 2017, and our first patient was treated in May 2017.
While the landmark trial (Argus II Retinal Stimulation System Feasibility Protocol, NCT00407602) [1‒3] mainly focused on the implant’s safety and efficacy, subsequent studies from different centers reported various anatomical and functional outcomes [4‒6]. Herein, we present the anatomic and functional outcomes of the first series of Korean RP patients who regained sufficient vision to read letters and achieved a certain degree of independent mobility after retinal prosthesis implantation.
Methods
Patient Selection
We included 5 patients who underwent retinal prosthesis implantation between May 2017 and April 2018 by one surgeon (Y.H.Y.) and were followed for over 12 months. All of the patients were screened and included based on Argus II Korean Conformity-approved indications. The inclusion criteria were: age 25 years or older, diagnosis of RP, and visual acuity (VA) of “light perception” or “hand motion.” We were careful not to include any patient who could use their residual visual function to read letters. All of the subjects previously had normal vision and were willing to be followed-up at regular visiting schedules and participate in postoperative vision rehabilitation. The exclusion criteria were as follows: too short (<20.5 mm) or too long (>26.0 mm) an axial length, ocular diseases concurrent with RP, and physical contraindications for device implantation such as systemic illnesses or the presence of cochlear implants.
We performed a complete ophthalmologic examination for each patient, including a review of the medical and clinical history, measurement of VA, slit-lamp biomicroscopy, a dilated fundoscopic examination, spectral-domain optical coherence tomography (SD-OCT), visual field testing, and full-field electroretinogram (ERG), and we diagnosed them with late stage RP. To determine the ability to perceive light, we performed a photo-flash test. In addition, we evaluated inner retinal function using electrical stimulation of the whole globe by measuring the transcorneal electrical evoked response (EER) [7‒9]. We excluded patients with a high EER threshold or no perception of corneal electrical stimulation. In detail, a sterile single-use ERG jet electrode (Fabrinal SA, Switzerland) was attached to the cornea, and a commercially available neurostimulator was used (MEE 1,000 stimulator; Nihon Koden, Japan) for stimulation. Rectangular biphasic current pulses (1-ms positive, directly followed by 1-ms negative) were applied. The electrical phosphene thresholds were measured 3 times, and their average was used for the EER results. The EER threshold was measured by escalating the intensity of the EER stimulation until the patient felt pain, which was at 7 mA in most cases, and only the cases with an EER result of 4 mA or less were included.
Based on the aforementioned screening test results, we selected the eye for retinal prosthesis implantation in each patient.
Surgical Procedures, Program Activation, and Rehabilitation Training
We performed the surgery as previously described [1], with some modifications. After performing a 360-degree conjunctival peritomy and isolating the rectus muscles, we sutured the receiver coil and electronic case at the predetermined location in the superotemporal quadrant. Following a core vitrectomy, triamcinolone was injected to help the surgeon perform a posterior vitreous detachment. Intraoperative OCT confirmed separation of the thin adherent cortical vitreous from the retina and its complete removal from the macular area, where we would place the microelectrode array. When the epiretinal membrane was present, we carefully peeled it but left the internal limiting membrane intact. We performed an intraoperative OCT while placing the array on the macula to ensure close proximity of the array to the underlying retina. After positioning the array properly, we tacked it down to the sclera using a retinal tack. We tested the impedance of each electrode of the implanted array by connecting it to the Clinician Fitting System (Second Sight Medical Products, Inc.). After confirming the integrity of the array, we meticulously closed the scleral incisions and conjunctiva.
Two to 3 weeks after the implantation surgery, we performed a fitting process for the device in each patient at the clinic. After measuring the threshold for each electrode on the array for the minimum amount of electrical current required to create perception, we created customized settings on a visual processing unit. Subsequently, we activated the camera in the glasses for the first time, enabling the patient to perceive a real-time video image of the surroundings using array stimulation.
Patients returned to the clinic every 2–4 weeks for specialized artificial vision rehabilitation. First, we comprehensively investigated the profile of the patients’ motivation and expectations and assessed their visual ability encompassing meaningful uses of vision in daily activities. Then, customized training sessions were provided. In the early sessions, we trained the patients to be aware of the eye, head, and camera position for locating light sources, discerning luminance, and recognizing shapes. In the later sessions, we focused on training the patients to enhance their newly acquired visual sense of orientation and mobility in the activities of daily living.
SD-OCT Imaging
We evaluated the patients on the first postoperative day and 1, 3, 6, and 12 months postoperatively. Experienced technicians obtained the SD-OCT images using the Spectralis OCT system (Heidelberg Engineering, Heidelberg, Germany). Two retina specialists (Y.H.Y. and Y.J.K.) selected and analyzed the images independently. We performed the following assessments of the macular structure and spatial relationship between the array and the underlying retina using a raster scan protocol centered at the fovea covering the electrodes.
Mean Electrode-to-Retina Distance
For 60 electrodes, we measured the vertical gap between each electrode and the inner limiting membrane of the retina using embedded calipers, as described previously [4]. Among 1,200 electrodes (60 electrodes of 5 implants at 4 follow-ups), we could measure the gap for 1,061 (88.4%). For the remaining 139 electrodes (11.6%), OCT showed a poor image quality or did not cover the implanted area, and we could not perform the assessments.
Retinal Morphologic Changes
We evaluated the available B-scans through the array at each visit to detect any preretinal hyperreflective membranes. In cases of presence of the preretinal membrane, we measured the thickness (vertical thickness between the upper and lower surfaces of the fibrosis) and the extent (number of electrodes overlying the fibrosis) of the fibrosis underneath the electrode. In addition, in cases of occurrence of retinoschisis, we evaluated its onset, duration, and extent (number of electrodes overlying the pathologic lesions).
We evaluated the onset, duration, and extent of cystoid macular edema if present. We also measured the retinal thickness in the B-scan from 4 locations along the long axis of the implant preoperatively and 1, 3, 6, and 12 months postoperatively. We performed an analysis of the average of the measurements.
Visual Function Tests
Computer-Based Visual Function Test
We tested square localization (SL) and direction of motion (DM), developed by Second Sight Medical Products (SSMP, Inc., Sylmar, CA, USA) for objective assessment of basic visual skills to cover the range of low-vision restored using the retinal implant [1‒3]. We performed these tests preoperatively and 3, 6, and 12 months postoperatively. We recorded the mean error (cm and degrees, retrospectively) and correct responses for analysis.
Early Treatment Diabetic Retinopathy Study and Korean Letter Reading
To assess the functional vision for micro-scanning, we used the smallest letters on the 4-m Early Treatment Diabetic Retinopathy Study (ETDRS) chart at a distance of 50 cm in the follow-ups at 3, 6, and 12 months postoperatively. We also tested whether the patients were able to read Korean letters.
Functional Low-Vision Observer-Rated Assessment
To assess the functional vision for macro-scanning in daily activities, we conducted Functional Low-Vision Observer Rated Assessment (FLORA) preoperatively and at 12 months postoperatively. FLORA consists of 3 parts, i.e., a self-report of the experience with the retinal prosthesis, an objective assessment of the performance of visual tasks, and a comprehensive report on the effect of the retinal prosthesis. In this study, we mainly analyzed the performance of visual tasks, orientation and mobility tasks, activities of daily living, and social interactions.
Safety
We performed complete ophthalmologic examinations in each patient, including measurement of the intraocular pressure, slit-lamp biomicroscopy, a dilated fundoscopic examination, and SD-OCT. Based on these, we evaluated the presence of possible postoperative complications reported in the landmark clinical trials [1‒3] (i.e., conjunctival erosions, hypotony, conjunctival dehiscence, endophthalmitis, retinal detachment or break, and ocular infectious conditions).
Results
The retinal prosthesis was successfully implanted in all 5 patients (2 males and 3 females). Table 1 shows the baseline demographics and characteristics of the participants. The mean (±SD) age was 56.6 ± 3.9 years. VA was “light perception” in both eyes in 4 patients and “hand motion” in 1 patient. The mean electrical phosphene threshold was 2.5 ± 0.8 mA with an electrical pulse in the implanted eye for 1 ms. Patient 2 had resting nystagmus, and patient 3 had an epiretinal membrane that was peeled during the surgery. All of the patients had a pseudophakic lens in the implanted eye. Preoperatively, SD-OCT revealed extensive loss of the photoreceptor layer, including the macular area, in all of the eyes. All of the eyes had a flat posterior pole without staphyloma, but patient 2 had a slightly steep macular concavity.
Anatomic Outcomes
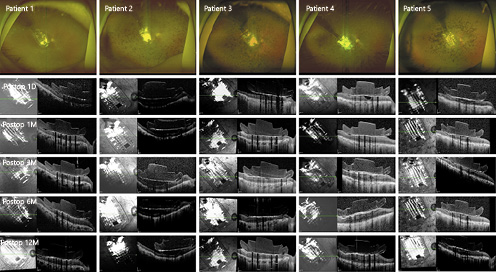
In all of the eyes, the microelectrode array was well positioned over the macula. We did not observe optic disc-to-array overlap in any eye at any postoperative visit (Fig. 1).
Postoperative fundus photographs and OCT images of the patients. In all of the eyes, the microelectrode array was well positioned over the macula. OCT revealed the development of fibrosis-like hyperreflective tissues at the interface between the array and the retina in 3 eyes (first seen in patient 1 at 12 postoperative months and in patients 3 and 4 at 3 postoperative months).
Postoperative fundus photographs and OCT images of the patients. In all of the eyes, the microelectrode array was well positioned over the macula. OCT revealed the development of fibrosis-like hyperreflective tissues at the interface between the array and the retina in 3 eyes (first seen in patient 1 at 12 postoperative months and in patients 3 and 4 at 3 postoperative months).
Mean Electrode-to-Retina Distance
Postoperatively, the array was completely apposed to the retinal surface in 4 patients and remained in good contact with the underlying macula for 12 postoperative months. The mean distance of between the bottom of the implant and the top of the retinal surface for 898 out of 1,000 electrodes of 4 patients (patients 1, 3, 4, and 5) was 9.2 µm (range 0.0–131.5). In patient 2, who had a preexisting macular concavity, the mean distance at 1 postoperative month (288.8 µm) remained stable over 12 postoperative months (282.1 µm; Fig. 1, 2a).
a Postoperative gap between the electrode and the retina. Patient 2, who had a preexisting macular concavity, showed a considerable mean gap, which remained stable over 12 months. The array was completely apposed to the retinal surface in the other 4 patients. b Thickness of the fibrosis at the midline. c Retinal thickness at baseline, and postoperative periods. After retinal fibrosis-like hyperreflective tissues developed, the retinal thickness tended to increase over time.
a Postoperative gap between the electrode and the retina. Patient 2, who had a preexisting macular concavity, showed a considerable mean gap, which remained stable over 12 months. The array was completely apposed to the retinal surface in the other 4 patients. b Thickness of the fibrosis at the midline. c Retinal thickness at baseline, and postoperative periods. After retinal fibrosis-like hyperreflective tissues developed, the retinal thickness tended to increase over time.
Retinal Morphologic Changes
OCT revealed the development of fibrosis-like hyperreflective tissues at the interface between the array and retina in 3 eyes, with an onset period ranging from 3 to 12 months after implantation (Fig. 1, 2b; Table 2). After the occurrence of retinal fibrosis-like tissues, the retinal thickness tended to increase over time (Fig. 2c).
Retinal morphological changes and number of functioning electrodes after Argus II implantation and their extent

Functional Outcomes
Computer-Based Visual Function Test
While patients with a poor baseline visual function (patients 1, 2, and 5) showed notable improvements, those with a high percentage score in baseline SL/DM (patients 3 and 4) failed to show further improvement postoperatively, perhaps because of the ceiling effect. When averaged, patients’ accuracy at localizing the square appeared to be better when the Argus II was on. However, patients’ accuracy at identifying the direction of movement was not substantially improved when the Argus II was on (Fig. 3a, b).
Graphs showing the mean error for SL (a) and for identifying the DM (b) preoperatively (baseline; BL) and at 3, 6, and 12 months postoperatively. When the Argus II was on compared to it being switched off, patients’ accuracy appeared to improve in SL but not in DM.
Graphs showing the mean error for SL (a) and for identifying the DM (b) preoperatively (baseline; BL) and at 3, 6, and 12 months postoperatively. When the Argus II was on compared to it being switched off, patients’ accuracy appeared to improve in SL but not in DM.
ETDRS Letter Reading and Korean Letter Reading
All of the patients could read ETDRS letters ranging from the 1st to the 6th line at a distance of 50 cm. This improvement was noted at 3 months postoperatively in 4 patients and at 12 months in 1 patient. Three patients (patients 1, 3, and 4) could read Korean words and 1 patient (patient 1) could write simple Korean sentences (video clip in real time; online suppl. video 1; see www.karger.com/doi/10.1159/000513585 for all online suppl. material).
Functional Low-Vision Observer Rated Assessment
In all of the patients, FLORA showed significant improvement in multiple tasks of visual orientation and mobility, daily life, and social interactions (Fig. 4). Patients demonstrated profound progress in recognizing objects, locating shapes for orientation, and walking independently with visual clues (video clip in real time; online suppl. video 1).
Results of the FLORA. We compared the preoperative and 1-year postoperative degrees of difficulty in performing a range of activities. Patients demonstrated profound progress in recognizing objects and walking independently with visual clues.
Results of the FLORA. We compared the preoperative and 1-year postoperative degrees of difficulty in performing a range of activities. Patients demonstrated profound progress in recognizing objects and walking independently with visual clues.
Complications
During 1 postoperative year, none of the patients experienced any device- or surgery-related complications such as hypotony, conjunctival erosions or dehiscence, tack displacement, retinal detachment, or endophthalmitis. All of the implants remained in good condition.
Discussion
In this study, we report the outcomes of retinal prosthesis implantation in a case series of advanced RP patients in Korea. Since 2007, approximately 300 cases of Argus II implantation have been recorded worldwide, reporting various anatomical and functional outcomes [1‒3]. Surgical outcomes continue to improve as surgeons learn more and have a better understanding of this new technology.
Although the number of patients was only 5, all of them could read letters, identify simple objects, and move independently in a familiar environment with the retinal implant. Anatomically, the array was in close proximity to the retinal surface in all of the patients, and the array position remained stable throughout the follow-up period. None of them showed any of the previously reported intra- or postoperative complications.
Although we need more studies to identify the visual predictors following implantation, the improvements in anatomical and functional outcomes in this study are likely due to the following factors: (1) careful patient selection, considering the integrity of the inner retinal layer and the visual pathway; (2) accurate surgical procedure, involving placement of the microelectrode array on the underlying retinal surface; and (3) optimal rehabilitation training [7].
First, we selected patients with a relatively healthy inner retinal layer and visual pathway. In clinical practice, there are no definitive measures to assess the integrity of the inner retinal layer and visual pathway. Based on previous studies [1, 3, 7], we selected eyes with a preserved ganglion cell layer in the macular area on SD-OCT. Postmortem histological studies revealed that the bipolar and retinal ganglion cell layers remained relatively unaffected, with a preservation rate of 78 and 30%, respectively, even in patients with severe RP [10]. In addition, we performed a test of retinal activation using transcorneal electric stimulation (TcES). We used the ERG jet (Fabrinadal SA) to deliver electrical current evenly to the eye and measured the phosphene threshold during TcES [9]. All of our patients responded to the electrical stimulation, with relatively low thresholds.
While the US guidelines allow patients with a VA of bilateral light perception or worse, we followed the European Union (EU) guidelines, which allow patients with bilateral hand motion or worse. As most patients with no light perception failed to respond to TcES, suggesting a poor integrity of the visual pathway, we excluded those patients from the study.
Second, correct placement of the microelectrode array on the underlying retinal surface was critical. As the microelectrode array was made of a polyamide material and had a size of 9.0 × 5.5 mm [11], the contour of the posterior pole had to be sufficiently flat. As the posterior pole of the end-stage RP eyes tended to show a steep contour compared to normal eyes [12], a careful preoperative SD-OCT evaluation was needed. In addition, a special effort was dedicated to the surgical procedures, including meticulous measurement for localization of the periocular components. Previous studies have shown that the stimulation thresholds closely correlated with the distance between the array and retinal surface [13, 14]. To achieve optimal placement of the microelectrode array on the macular surface, we used intraoperative OCT in all of the cases. Rachitskaya et al. [15] published a case report utilizing intraoperative OCT during implantation of Argus II. Intraoperative OCT was extremely useful in monitoring the placement of the array on the posterior pole and in confirming the absence of a gap between the implant and retinal surface.
Moreover, we used an intraoperative OCT to identify the thin adherent cortical vitreous, induce posterior detachment, and remove the vitreous body completely from the implanted retinal surface. RP eyes have a thin adherent vitreous cortex as a secondary change of vitreous degeneration [16]. Incomplete removal of the cortical vitreous was likely to result in a gap between the array and retinal surface and result in postoperative complications, such as preretinal fibrotic membrane, retinoschisis, and tractional retinal detachment [4].
While previous studies have mainly focused on the implant’s safety and efficacy, a few studies have reported the anatomical outcomes. In a study conducted in France [17], patients showed variable distances between the array and the retina, with only a few patients showing full apposition. Recently, an international post-marketing surveillance study on 33 patients at 16 surgical sites [6] revealed that the arrays were in complete contact with the macula in about half of the eyes after 1 year.
Another postoperative retinal change was the development of fibrosis-like hyperreflective tissues at the interface between the array and retinoschisis. Rizzo et al. [4] reported that, among the eyes of 20 patients who were followed-up for 36.8 (±19.4) months, 10 implanted eyes developed a membrane and 9 progressed to retinoschisis. An international post-marketing surveillance study also reported that 89% (16 out of 18) of the eyes had some degree of macular thickening or cystoid macular edema by 12 postoperative months, which may be because of long-term mechanical or electrical stimulation of the array [6].
In our series, 4 out of 5 eyes showed complete apposition of the array to the underlying macula intraoperatively and at 12 months of follow-up. One patient with a slightly steep macular concavity had a persistent gap of <300 μm from month 1 to month 12 but showed continuous improvement in visual function in both computer-based visual tests and FLORA. While 3 eyes developed a membranous structure in 3–12 postoperative months, the membrane was thinner than 50 μm in 1 eye, and only one of them progressed to retinoschisis, probably because of tractional forces from the membrane [4]. The patient who developed diffuse cystoid macular edema developed retinal thickening during 3–12 postoperative months, but the visual function did not seem significantly affected by these retinal morphologic changes. We hypothesize that the development of epiretinal fibrosis may be induced by direct array-to-retina contact, and the fact that no membrane formation was observed in patient 2 also supports this hypothesis. We noted the membrane structure around the tack site in the early stage of membrane formation. Based on these findings, we speculate that the fibrosis-like membrane would be developed from the direct contact area of the tack irritation to the retina with the cellular ingrowth.
Third, optimal rehabilitation training is important for a good functional outcome. Although there is no consensus on vision rehabilitation for a retinal prosthesis, we followed the protocols described in the SSMP rehabilitation guide prepared specifically for the recipients of Argus II, [18, 19] and we adopted the general principles of low vision. We performed about 20 sessions of training, including light localization, tracking, shape recognition, luminance discrimination, visual integration, orientation, and mobility. For the maximal orientation and mobility for distant work, patients utilized a cane together with Argus II at all times. Because Argus II has only 15–20 degrees of visual field and it does not have true stereoscopic vision, for the Argus II recipient is hard to know how far away the distant target is. Patients may obtain a pseudosense of depth perception if they are mindful of how high they are holding their head, and watch the object steadily become larger in their visual field as they draw closer. In order to use the Argus II to follow a distant target without losing it from their field, a cane is essential to manage obstacles at their immediate front. As this training process requires patients’ active participation, only those who are motivated to adhere to postoperative rehabilitation should be selected.
All of our patients could read ETDRS letters, and 3 could read letters on the fourth line of the ETDRS chart at 50 cm or closer. da Cruz et al. [20] reported that 4 out of 28 Argus II patients could read English letters measuring 0.9 cm (1.7 degrees) at a distance of 30 cm, correctly identify unrehearsed 4-letter words, and read English letters measuring 1.3 cm (2.5 degrees) at a distance of 30 cm. It is noteworthy that our patients could read Korean words and that 1 patient could write simple Korean sentences. The Korean alphabet, known as Hangul, is typographically more complex because its letters are printed in syllable blocks, resulting in up to 4 graphemic elements per syllable [21]. Thus, the performance of reading and writing Korean letters may be considered as a significant improvement.
Regarding functional vision assessment, all of the patients showed significant improvement. FLORA was developed to obtain an objective assessment of the utilization of a retinal prosthesis by the patient in a series of 35 daily activities associated with orientation, mobility, daily living, and social interaction [21]. Our patients showed a significantly better performance in multiple tasks compared to the results of the Argus II feasibility trial, in which patients were able to complete 24 of 35 tasks (69%) with greater ease when the device was on [1].
Safety was a primary outcome measure of the pivotal feasibility trial [1‒3]. During a range of 5.2–7.4 years, 3 of the 30 enrolled patients were explanted because of recurring conjunctival erosion, 4 developed hypotony, and 2 required retack. In addition, 2 had failed because of a gradual loss of the ability to maintain a radiofrequency link between the transmitting and receiving coils [1]. Recently, a study by Rizzo et al. [22] reported that in 12 months after surgery, no major complications were detected, except for a medically treated increased intraocular pressure and mild choroidal detachment. None of our patients developed any of the previously reported complications during the surgery or in the postoperative period. The meticulous measurement and precise surgical skill in each step likely prevented the implanted eyes from developing serious complications, such as hypotony, conjunctival dehiscence, retinoschisis, or displacement of the array [23].
Our study has several limitations, i.e., the small number of patients and the relatively short follow-up period. Another limitation of this study is that we could not use the standardized method in testing letter-reading ability. As there is still no standard method for patients with a retinal prosthesis, a direct comparison of letter-reading ability between previous studies and our study would not be fair. However, the strengths of our prospective, consecutive case series are that all of the patients were followed-up and that they showed a significant improvement in letter reading ability, mobility tasks, and daily life tasks, without any safety issue.
In conclusion, Argus II retinal prosthesis implantation allowed Korean patients with advanced RP to read ETDRS and Korean letters and to show improvements in real-life performances. Careful preoperative evaluation, accurate operative procedures, and specialized rehabilitation seemed to be the key in achieving the maximal effect of the Argus II preretinal implant.
Statement of Ethics
This study was performed with approval from the Institutional Review Board of the Asan Medical Center (Seoul, Korea; IRB No. 2017-0420) and in accordance with the tenets of the Declaration of Helsinki. All of the patients provided signed informed consent.
Conflict of Interest Statement
Y.H.Y. and Y.J.K. have no affiliations with or involvement in organizations or entities with financial or nonfinancial interests in the subject matter or materials discussed in this paper. M.H. has intellectual property rights in Argus technology and is equity owner and has patents and royalties in Second Sight Medical Products, Inc., Sylmar.
Funding Sources
This study was supported by a grant from the Asan Medical Center. The funding organization had no role in the design or conduction of this research.
Author Contributions
Y.H.Y.: conception of this study, acquisition and analysis of data, and writing and critical revision of this paper. M.H.: conception, writing, and critical revision of this paper. Y.J.K.: acquisition and analysis of data and writing and critical revision of this paper. All of the authors gave their final approval.
References
Additional information
This work was presented in part as a video presentation at the American Academy of Ophthalmology (AAO) Annual Meeting on October 25–26, 2018, in Chicago, IL, USA.