Abstract
Voxel-based morphometry (VBM) has become an increasingly common method for assessing neuroanatomical asymmetries in human in vivo magnetic resonance imaging (MRI). Here, we employed VBM to examine asymmetries in white matter in a sample of 48 chimpanzees (15 males and 33 females). T1-weighted MRI scans were segmented into white matter using FSL and registered to a common template. The segmented volumes were then flipped in the left-right axis and registered back to the template. The mirror image white matter volumes were then subtracted from the correctly oriented volumes and voxel-by-voxel t tests were performed. Twenty-seven significant lateralized clusters were found, including 18 in the left hemisphere and 9 in the right hemisphere. Several of the asymmetries were found in regions corresponding to well-known white matter tracts including the superior longitudinal fasciculus, inferior longitudinal fasciculus and corticospinal tract.

Introduction
Evidence of neuroanatomical asymmetries in the human brain has a long history dating back to the early observations of Dax, Broca and Wernicke [Nishitani et al., 2005; Cooper, 2006]. Early reports of neuroanatomical asymmetries were largely anecdotal but in recent times, the mounting data have overwhelmingly shown evidence of asymmetry in structure and function in the human brain [Corballis, 2002; Hammond, 2002; Hatta, 2006]. Most studies of neuroanatomical asymmetries in the human brain have used region-of-interest approaches with an emphasis on quantification and comparison of gyri or sulci between hemispheres. That is to say, specific regions are defined by sulci or other neuroanatomical landmarks and the regions are quantified in the left and right hemispheres of the brain. For example, there are well-documented neuroanatomical asymmetries in the human planum temporale and the frontal operculum, regions known to be involved in higher cognitive processes including language and speech [Corina et al., 1992; Galaburda, 1995; Beaton, 1997; Foundas et al., 1998; Shapleske et al., 1999; Josse et al., 2003; Keller et al., 2007; Keller et al., in press].
More recently, with the advent of high-resolution magnetic resonance imaging (MRI), investigators have begun to assess asymmetries of the entire brain using voxel-based morphometry (VBM) [Good et al. 2001; Watkins et al. 2001; Hervé et al. 2006]. With VBM, MRI scans are aligned to a common spatial template and the distribution of gray and white matter across the entire brain is quantified to assess consistency across subjects based on the contiguous clustering of tissue concentration in different regions.
One advantage of VBM is that it provides an opportunity to consider lateralization in white matter, a much overlooked dimension of neural organization that some have argued is the foundation for both the development and evolution of functional asymmetries and higher cognitive functions [Ringo et al., 1994; Rilling and Insel, 1999a, b; Aboitiz et al., 2003; Schoenemann et al., 2005; Schoenemann, 2006; Barrick et al., 2007] . There are a number of well-known white matter tracts in the brain that connect cortical regions and recent studies suggest that some of these tracts are lateralized [Highley et al., 2002; Kier et al., 2004; Catani and Ffytche, 2005; Catani et al., 2005]. For instance, the frontal operculum and posterior temporal regions are connected by a white matter tract referred to as the ‘arcuate fasciculus’. Clinical studies suggest that lesions to the arcuate fasciculus pathway can lead to conduction aphasia, which is an inability to repeat speech. In the classic model of language lateralization, with conduction aphasia, patients can comprehend the requested speech actions but are unable to send those signals to the motor areas of the frontal operculum to execute the actions [Glasser and Rilling, 2008]. Although the presence of the arcuate fasciculus has been known for quite some time, it was not until recently that studies have found it to also be lateralized to the left hemisphere in humans. Specifically, recent studies using diffusion tensor imaging (DTI) have documented leftward asymmetries in the volume and extent of connections of the arcuate fasciculus in the human brain [Nucifora et al., 2005; Glasser and Rilling, 2008; Rilling et al., 2008]. Thus, the functional and anatomical asymmetries seen in the frontal operculum and the posterior temporal lobe in the human brain are similarly connected by an asymmetric white matter tract, although some have suggested that the arcuate fasciculus asymmetry is unrelated to functional lateralization for language [Vernooij et al., 2007]. In addition to tracts, there is also evidence of asymmetries in the volume of white matter underlying specific cortical regions such as the pre- and postcentral gyri, inferior frontal gyri and prefrontal cortex, and these have been linked to both subject sex and handedness [Paus et al., 2001; Buchel et al., 2004; Raz et al., 2004; Hervé et al., 2006; Barrick et al., 2007; Gur et al., 2007].
Although VBM does not reveal white matter tracts in the manner that is accomplished by DTI, it does provide a means to identify regions within the brain where white matter asymmetries are prevalent. The purpose of the current study was to use VBM to determine whether chimpanzees show asymmetries in white matter. Within the past 10 years, a number of studies have identified population level asymmetries in great apes, particularly chimpanzees, using region-of-interest approaches. These include the planum temporale [Gannon et al., 1998; Cantalupo et al., 2003], planum parietale [Gannon et al., 2005; Taglialatela et al., 2007], and the inferior frontal gyrus [Cantalupo and Hopkins, 2001; Hopkins and Cantalupo, 2004; Hopkins et al., 2008]. More recently, VBM has also been used to assess gray matter asymmetries in chimpanzees [Hopkins et al., 2008]. However, few studies have yet considered white matter asymmetries in nonhuman primates. In this study, we sought to determine whether white matter asymmetries were evident in the chimpanzee brain, and examine whether or not they correspond to regions where known white matter tracts are present in the human brain.
Materials and Methods
Materials
MRI scans were obtained from a sample of 48 captive chimpanzees (15 males and 33 females). All the chimpanzees were members of a captive colony housed at Yerkes National Primate Research Center (YNPRC) in Atlanta, Ga., USA. The subjects ranged in age from 6 to 42 years (mean = 22.31, SD = 11.45).
Image Collection and Procedure
Subjects were first immobilized by telazol injection (2–6 mg/kg) and subsequently anesthetized with propofol (10 mg/kg/h) following standard procedures at the YNPRC. Subjects were then transported to the MRI facility. The subjects remained anesthetized for the duration of the scans as well as the time needed to transport them between their home cage and the imaging facility (total time about 2 h). Subjects were placed in the scanner chamber in a supine position with their head fitted inside the human-head coil. Scan duration ranged between 40 and 50 min. The chimpanzees were scanned using a 3.0 T scanner located at the YNPRC (Siemens Trio; Siemens Medical Solutions USA, Inc., Malvern, Pa., USA). T1-weighted images were collected using a three-dimensional gradient echo sequence (pulse repetition = 2,300 ms, echo time = 4.4 ms, number of signals averaged = 3, matrix size = 320 × 320). After completing MRI procedures, the subjects were temporarily housed in a single cage for 6–12 h to allow the effects of the anesthesia to wear off, after which they were returned to their home cage. The archived MRI data were transferred to a PC running Analyze 8.0 (Mayo Clinic, Mayo Foundation, Rochester, Minn., USA) software for postimage processing.
Image Normalization and Registration
Once the images were acquired, the skulls were removed virtually, and each subject’s brain was coregistered to a template of a chimpanzee brain [Rilling et al., 2007]. The chimpanzee brain template was created using a two-tiered procedure. Initially, the MRI scans of 8 chimpanzees (subjects included in this study) were placed in stereotaxic orientation using AFNI software and then averaged together into a single image. Next, each individual MRI scan was spatially normalized to this template using an affine transformation. Subsequently, all the spatially normalized MRI scans were averaged to create the template. Each individual MRI scan was coregistered to the template described above using three-dimensional voxel registration with a linear transformation (Analyze 8.0, Mayo Clinic). The MRI scans were then segmented into gray, white and cerebrospinal fluid tissue using FSL 4.1 (Analysis Group, FMRIB, Oxford, UK) [Smith et al., 2004].
For the VBM analysis, an average white matter template was constructed from the entire sample of MRI scans by averaging all of the segmented white matter volumes together. Each individual segmented white matter volume was then registered to this white matter template. Next, each subject’s white matter volume was then flipped 180° in the left-right axis to create a mirror image brain volume (fig. 1), and each of these flipped volumes was then reregistered to the white matter chimpanzee template. For each ape, the mirror image white matter volumes were then subtracted from the correctly oriented volumes (fig. 1). An average difference volume was created from all of the individual difference volumes, and this volume was lowpass filtered with a 5-mm isotropic kernel. Voxel-by-voxel t tests were calculated and significant clusters were identified as 3 or more contiguous voxels on three or more consecutive 2-mm slices in the axial plane with intensity values ≥6.00 (i.e. 9 total voxels; 72 mm3). Significance levels of the observed t values were set at t ≥ 6.00 (p < 0.0001) to adjust for multiple comparisons. Anatomical identification of white matter structures followed the atlas of human white matter by Wakana et al. [2004].
Sagittal views of the left (LH) and right (RH) hemispheres at different planes of depth. The color bar indicates the variation in t values corresponding to asymmetries in the different white matter clusters.
Sagittal views of the left (LH) and right (RH) hemispheres at different planes of depth. The color bar indicates the variation in t values corresponding to asymmetries in the different white matter clusters.
Results
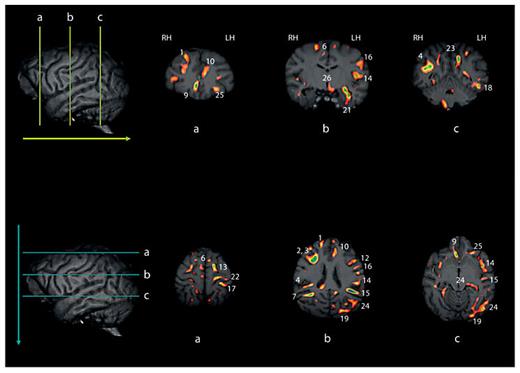
White matter asymmetries projected onto a 3D chimpanzee brain at different parasagittal levels are shown in figure 1. As can be seen, there are pronounced leftward asymmetries, particularly within the temporoparietal and frontal regions. In total, 27 significant clusters were identified by the VBM analysis. The regions and their x, y, z coordinates are shown in table 1. Leftward asymmetries were found for 18 regions and rightward asymmetries were found for 9 regions. Within the left hemisphere, asymmetries were present in several main gyri including the occipital gyri (clusters 19, 20), angular gyrus (cluster 24), postcentral gyrus (clusters 16, 17), precentral gyrus (clusters 12, 22) and the middle and superior frontal gyri (clusters 11, 13). Several white matter asymmetries were also found in regions corresponding to well-know tracts within the human brain including the superior longitudinal fasciculus (clusters 14, 18), inferior frontooccipital fasciculus (clusters 15, 21), inferior longitudinal fasciculus (cluster 18) and uncinate fasciculus (cluster 21). Within the right hemisphere, asymmetries were also found in superior and middle frontal gyri (clusters 1, 6) as well as in the inferior frontal gyrus (cluster 3), and superior parietal lobe (cluster 5). Asymmetries in tracts corresponding to the superior longitudinal fasciculus (clusters 3, 4) and inferior frontooccipital fasciculus (cluster 7) were also found within the right hemisphere fig. 2.
Upper and lower panels reflect selective coronal (upper) and axial (lower) views of the different white matter cluster revealed by VBM. The numbers in each corresponding image reflect the cluster identified in table 1. Note, some clusters are unlabeled and in these cases, the clusters are visible on the single slice shown in the image, but the size of the cluster when considered across multiple slices may not have reached our size criterion (see text for description). LH = Left hemisphere; RH = right hemisphere.
Upper and lower panels reflect selective coronal (upper) and axial (lower) views of the different white matter cluster revealed by VBM. The numbers in each corresponding image reflect the cluster identified in table 1. Note, some clusters are unlabeled and in these cases, the clusters are visible on the single slice shown in the image, but the size of the cluster when considered across multiple slices may not have reached our size criterion (see text for description). LH = Left hemisphere; RH = right hemisphere.
Discussion
Chimpanzees showed a number of population level asymmetries in white matter and a number of the clusters revealed in the analysis correspond to regions where well-known white matter tracts are present in the human brain including the superior longitudinal fasciculus, inferior frontooccipital fasciculus and the uncinate. Notably, leftward asymmetries were prominent in the more lateral brain regions and this finding is consistent with the report by Cantalupo et al. [2009] in chimpanzees showing significant leftward asymmetries in the ratio of white-to-gray matter in perisylvian brain regions, particularly within the frontal lobe. The evidence presented here further supports the growing body of data documenting neuroanatomical asymmetries in nonhuman primates, especially chimpanzees, refuting claims that brain asymmetry is a uniquely human trait [Holloway and De La Coste-Lareymondie, 1982; Crow, 2004; Schoenemann, 2006].
White matter asymmetries were also found to underlie several prominant gyri, notably the pre- and postcentral gyri. Interestingly, there was also a leftward asymmetry in the corticospinal tract in our chimpanzee sample. The evidence of leftward asymmetries in the corticospinal tract, and post- and precentral gyri are consistent with at least one report of asymmetries in white matter of chimpanzees using DTI. Specifically, Longchuan et al. [2009] recently obtained DTI scans in a sample of 36 female chimpanzees and reported a significant leftward asymmetry in the tractography of the corticospinal system. Moreover, these authors found that the left corticospinal tract is less variable, and is located more posterior to the right corticospinal tract. Lastly, in terms of projections into the precentral gyrus, the left corticospinal tract is located more lateral compared with this tract in the right hemisphere in this sample of chimpanzees.
To what extent the white matter asymmetries reported here are the cause or consequences of early asymmetric input on to the motor and cognitive processes of chimpanzees is unclear. There is evidence that chimpanzees show population level behavioral asymmetries within the first month of life for head orientation, hand-to-mouth activities and grasping strength [Hopkins and Bard, 1993, 1995; Fagot and Bard, 1995] and the presence of these asymmetries may lead to the development of greater connectivity in the hemisphere contralateral to the preferred hand. There is also evidence that white matter increases during development as reflected in increasing size of the corpus callosum [Hopkins and Phillips, in press]. Unfortunately, the youngest ape we scanned was 6 years of age and there are no available published data on white matter development in chimpanzees and other great apes, particularly in very young apes. These data would be critical for assessing what role different experiential factors may or may not have on the development of white matter asymmetries in primates.
In summary, the evidence of leftward asymmetries in white matter in the chimpanzees, coupled with the data showing population-level right-handedness in many of these same apes, suggests that this association might be due to increased intrahemispheric connectivity in the hemisphere contralateral to the preferred hand. Whether these organizational differences are a consequence of the development of handedness or vice versa remains unclear, but warrants further investigation. Moreover, the specific white matter pathways that are associated with individual differences in behavioral asymmetries warrant further investigation using more precise in vivo imaging technologies, such as DTI. Based on the results reported here, we would hypothesize that significant leftward asymmetries will be found in chimpanzees and potentially other nonhuman primates.
Acknowledgements
This research was supported in part by NIH grants NS-42867 and HD-56232. The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. American Psychological Association guidelines for the ethical treatment of animals were adhered to during all aspects of this study and institutional animal care and use approval was obtained before conducting this work.