Abstract
Background: Conventional pulmonary rehabilitation programs improve exercise tolerance but have no effect on pulmonary function in patients with chronic obstructive pulmonary disease (COPD). The role of controlled breathing using respiratory biofeedback during rehabilitation of patients with COPD remains unclear. Objectives: To compare the effects of a conventional 4-week pulmonary rehabilitation program with those of rehabilitation plus controlled breathing interventions. Methods: A randomized controlled trial was performed. Pulmonary function (FEV1), exercise capacity (6-min walking distance, 6MWD), health-related quality of life (chronic respiratory questionnaire, CRQ) and cardiac autonomic function (rMSSD) were evaluated. Results: Forty COPD patients (mean ± SD age 66.1 ± 6.4, FEV1 45.9 ± 17.4% predicted) were randomized to rehabilitation (n = 20) or rehabilitation plus controlled breathing (n = 20). There were no statistically significant differences between the two groups regarding the change in FEV1 (mean difference –0.8% predicted, 95% CI –4.4 to 2.9% predicted, p = 0.33), 6MWD (mean difference 12.2 m, 95% CI –37.4 to 12.2 m, p = 0.16), CRQ (mean difference in total score 0.2, 95% CI –0.1 to 0.4, p = 0.11) and rMSSD (mean difference 2.2 ms, 95% CI –20.8 to 25.1 ms, p = 0.51). Conclusions: In patients with COPD undergoing a pulmonary rehabilitation program, controlled breathing using respiratory biofeedback has no effect on exercise capacity, pulmonary function, quality of life or cardiac autonomic function.
Introduction
Expiratory flow limitation in patients with chronic obstructive pulmonary disease (COPD) results from progressive airway inflammation causing parenchymal destruction, mucosal oedema, airway remodelling, mucous impaction and increased cholinergic airway smooth muscle tone [1]. Advanced COPD is associated with reduced exercise tolerance and daily physical activity resulting in impaired health-related quality of life [2], high health care use [3] and increased mortality [4]. Furthermore, patients with advanced COPD have a pathological breathing pattern with enhanced intrathoracic pressure swings due to severe airway obstruction [5]. Previous studies have shown that pulmonary rehabilitation programs improve exercise tolerance [6,7] and peripheral muscle strength [8], reduce exacerbation rate [9] and improve health-related quality of life, but not pulmonary function in patients with COPD [10,11].
Predominantly diaphragmatic breathing (DB), pursed-lips breathing (PLB) and prolonged exhalation are the most commonly used controlled breathing techniques and the use of each of these modalities has certain clinical benefits. PLB appears to be an effective way to decrease dyspnoea [12], improve walking distance [13] and gas exchange [14,15]. DB in patients with COPD has been associated with improvement in blood gases [16]. However, the relative contribution of each of these modalities to the overall improvement of the patients is not clear. Previous studies assessing the effectiveness of controlled breathing included small numbers of heterogeneous patients and lacked a control group [15].
To address the question whether controlled breathing improves outcome of rehabilitation in COPD patients, we performed a randomized controlled trial (RCT) using respiratory feedback training (RBF) to assess the effects of a 4-week rehabilitation program using outcome parameters including pulmonary function tests, cardiopulmonary exercise capacity, health-related quality of life and cardiac autonomic modulation. We hypothesized that COPD patients who undergo pulmonary rehabilitation plus controlled breathing will benefit more than patients participating in conventional pulmonary rehabilitation classes.
Methods and Materials
Study Subjects
Patients with COPD referred to the Department of Respiratory Medicine, Ruhrlandklinik, University of Duisburg-Essen, Germany, between November 2008 and July 2009 were considered for participation in the study. Inclusion criteria were: clinically stable disease (no changes in medication dosage or frequency of administration, no clinical signs or symptoms of acute exacerbations and no hospital admissions in the preceding 6 weeks), age between 40 and 75 years, postbronchodilator FEV1 of less than 80% predicted and an FEV1/FVC ratio of less than 0.7, and a BMI of more than 18 and less than 25 kg/m2. We excluded patients with respiratory disorders other than COPD, α1-antitrypsin deficiency, a history of significant inflammatory disease other than COPD, cardiac diseases such as heart failure, cardiac arrhythmia and/or coronary artery disease, patients with a history of lung surgery, patients with diagnosis of cancer and patients who were unable to walk. Patients with oral corticosteroids and/or vasoactive medication at inclusion were also excluded from the study. Each participant signed and dated a written informed consent form prior to participation.
The study was approved by the Research Ethics Committee of the Medical Faculty, University of Essen-Duisburg, Germany (No. 07-3524) and written informed consent was obtained from all patients.
Study Design
We performed a RCT with sequential analysis of the clinical training effects; tests were performed prior to and following completion of cardiopulmonary exercise training.
Methods
Cardiopulmonary Exercise Training
Cardiopulmonary exercise training was performed according to publishedguidelines [17,18,19]. Participants attended the outpatient clinic 3 times per week (1.5-hour sessions) for 3–4 weeks performing 10 sessions of physical training. The session included dynamic strength training for the following muscles: quadriceps femoris, hamstrings, triceps surae, pectoralis major, deltoid, latissimus dorsi and triceps brachi. The dynamic strength training exercises were performed while seated. Patients started at 70% of their initial 1-repetition maximum (1RM) and fulfilled 3 cycles of 10 repetitions of isotonic muscle contractions [17,18,19] with a resting period of 2 min between the series. 1RM is the maximum amount of weight one can lift during a single repetition of a given exercise.
When the patients were able to perform 3 sets of 10 repetitions without any difficulty, effort was increased stepwise by 5% of the 1RM. Cardiopulmonary endurance training was performed on a cycle ergometer, using stepping exercises and arm cranking [17,18,19]. The initial intensity for cycling was set at 30% peak workload for 20 min. Increases in workload were based upon symptom scores.
Controlled Breathing Training
Twenty patients allocated to the RBF group participated in 10 supplemental 30-min sessions of controlled breathing using techniques of respiratory biofeedback training and were instructed on its performance during the first training session [20,21,22]. The respiratory biofeedback training system visually displays the desired respiratory pattern: the patients were trained to voluntarily improve their breathing pattern at rest for 10 min while seated with instructions for daily home practice. Furthermore, the patients were trained to improve their breathing pattern during the 20 min of cardiopulmonary endurance training which was performed on a cycle ergometer.
The patients were trained to modify four respiratory characteristics: rapid shallow breathing (increased respiratory rate and low tidal volume), breath-to-breath irregularity in rate and depth and predominant thoracic breathing. DB was performed as described by Gosselink [23,24] by ‘facilitating outward motion of the abdominal wall while reducing upper rib cage motion during inspiration’.
Dynamic hyperinflation occurs when inspiration commences prior to completion of the preceding exhalation so that air is trapped ‘upstream’ at the small bronchiolar and alveolar levels. To reduce dynamic hyperinflation, the patients were encouraged by prolonging the expiration prior to the initiation of the next breath while using PLB.
The breathing pattern was monitored by respiration sensors that measure the patient’s breathing rhythm at both umbilical and abdominal level. The respiration sensors were connected to an amplifier (Nexus-10™ medical device class IIa; TMS International BV, Enschede, The Netherlands) that converts the electrical impulses into acoustical and visual outputs. BioTrace+ software was used for physiological monitoring and signal processing.
The RBF training works with simple acoustic tones and visual graphic signals in order to inform the patient precisely about their actual breathing pattern. Both signals were simultaneously used as a feedback for the patient via an earphone and an overhead screen. Both signals increase and decrease in volume and intensity as the patient breathes in and out.
Measurements
Pulmonary Function
Spirometry, whole body plethysmography and diffusion capacity measurements were performed according to the American Thoracic Society and the European Respiratory Society guidelines with a commercially available system (Body 500™; ZAN, Oberthulba, Germany) [25]. Postbronchodilator spirometry was performed on the same day as the exercise tests (6-min walk test, 6MWT). Maximal inspiratory mouth occlusion pressure after 100 ms was measured as previously described [26].
Six-Minute Walk Test
All patients performed the 6MWT following pulmonary function testing. 6MWT distance was measured according to the guidelines of the American Thoracic Society [27,28]. Oxygen saturation and pulse rate were recorded using a standard pulse oximeter (Nexus-10™ medical device class IIa; TMS International BV). Additionally, scored sensations of breathlessness and leg fatigue were assessed using a modified Borg scale [29]. The 6MWT was performed on a 30-meter indoor track by an experienced investigator using standardized encouragement strategy [30], and the results were recorded in absolute values and in percent predicted [27]. To control for any learning effect, all patients performed two 6MWTs on two separate days, and the results of the second test were used for analysis.
Health-Related Quality of Life
The Chronic Respiratory Questionnaire (CRQ) is an established measure of health status for chronic obstructive pulmonary disease [31,32]. Previous studies have shown that an improvement in score in any domain of the CRQ of ≥0.5 represents the minimal clinically important difference that is noticeable to patients [31], changes in any domain of the CRQ >1.0 represent moderate improvements and changes in any domain of the CRQ >1.5 represent large improvements in health-related quality of life [32].
Cardiac Autonomic Function
Analysis of heart rate variability (HRV) is a powerful method to assess the autonomic nervous system. HRV is a physiological phenomenon describing the variation of the time interval between heart beats which is related to the relative acitivity of the sympathetic and parasympathetic nervous system. A reduced HRV implies an impaired ability of the heart to alter its own beat frequency and therefore a pathological condition of the cardiovascular system [33,37,38,39]. Cardiovascular autonomic function was assessed by measuring the standard time and frequency domain measures of HRV from 5 min of the R-R interval recordings in the ECG, with the patients in seated. HRV was obtained via 3-channel ECG recording (Nexus-10™ medical device class IIa; TMS International BV) as recommended by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [33]. To eliminate artefacts an automatic filter excluded RR sequence differing by more than 30% from the previous interval [33]. Time domain measures of HRV were calculated from the R-R interval tachograms. The following time domain measures of HRV were calculated: mean R-R (NN mean) in milliseconds, the standard deviation of R-R intervals (SDNN) in milliseconds and the root mean square successive difference of R-R intervals (rMSSD) in milliseconds.
The following standard frequency domain measures of HRV were computed: high-frequency spectral power (HF power, the density of the beat-to-beat oscillation in the R-R interval of HRV in the high-frequency band; HF = 0.15–0.4 Hz), low-frequency spectral power (LF power, the density of the beat-to-beat oscillation in the R-R interval of HRV in the low-frequency band; LF = 0.04–0.15 Hz), the ratio of LF to HF power (LF/HF ratio). LF/HF ratio has been described as a marker of sympathetic to parasympathetic balance [34,35,36]. Spectral components were expressed both as absolute values in milliseconds and as normalized units. To reflect the degree of parasympathetic modulation of heart rate, we used both HF power (%) and rMSSD (ms) [33,37,38,39].
Data Analysis
We performed an RCT (ClinicalTrials.gov identifier: NCT01175265) with sequential analysis of the clinical training effects; tests were performed prior to and following completion of cardiopulmonary exercise training. Twenty patients were assigned to receive 10 sessions of 1.5 h of physical exercise training plus additional ten 30-min RBF sessions (RBF group). In the other group, 20 patients were assigned to receive ten 1.5-hour sessions of physical exercise training only (control group) over 4 weeks. The technicians, who performed the pulmonary function tests and 6MWTs, were blinded for the randomization. In order to control for any residual learning effect, the patients performed two 6MWTs at baseline on separate days; the second 6MWT was used for analysis.
Data on pulmonary function, cardiopulmonary exercise capacity, health-related quality of life and cardiac autonomic modulation were collected prior to and following completion of 4 weeks of pulmonary rehabilitation.
A statistical software package was used for statistical analysis (SPSS for Windows, version 11.0; SPSS Inc., Chicago, Ill., USA). Descriptive data for continuous variables are expressed as mean ± SD. Effects of pulmonary rehabilitation were evaluated by within-group comparisons of changes over 4 weeks, using paired Student’s ttests. Differences in changes from baseline to follow-up between the two groups were compared using unpaired Student’s t tests. A p value of <0.05 was considered to be significant.
Results
Patients
Sixty-three patients with COPD (GOLD class I–IV) were evaluated for study participation. Forty-three patients were eligible for the study and agreed to participate. Twenty-two patients were randomized to the RBF group and 21 to the control group. Three patients withdrew from the study due to an acute COPD exacerbation during the training period.
Baseline anthropometrical characteristics and pulmonary function data of the 40 patients (23 females) with COPD are presented in table 1. Twenty-two patients had mild to moderate disease (GOLD I n = 4; GOLD II n = 18), and 18 patients had severe or very severe COPD (GOLD III n = 16; GOLD IV n = 2). There were no significant differences in the baseline anthropometrical characteristics and pulmonary function test results between the group receiving RBF and the control group.
Changes in Pulmonary Function, Cardiopulmonary Exercise Capacity and Health-Related Quality of Life
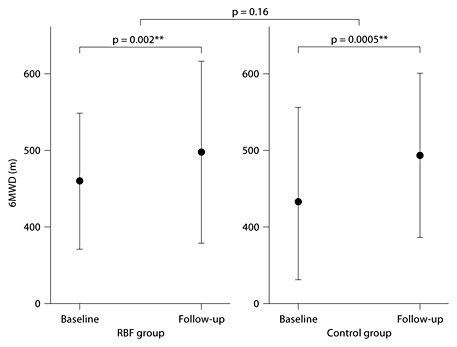
Between-group comparisons of changes in pulmonary function, health-related quality of life and cardiopulmonary exercise capacity over the 4 weeks of pulmonary rehabilitation are shown in table 2. There was no change in lung function test results during the 4-week program in any of the groups, and the groups did not differ (fig. 1).
Changes of pulmonary function,cardiopulmonary exercise capacity and health-related quality of life

There was no statistically significant difference in the change of FEV1 between the RBF group (left) and the control group (right) after the 4 weeks of pulmonary rehabilitation.
There was no statistically significant difference in the change of FEV1 between the RBF group (left) and the control group (right) after the 4 weeks of pulmonary rehabilitation.
Cardiopulmonary exercise capacity (6MWD) over the 4 weeks of pulmonary rehabilitation showed no significant differences between the groups (fig. 2). Within-group comparison for both study groups revealed a significant improvement in exercise capacity over the 4 weeks of pulmonary rehabilitation (fig. 2): the 6-min walking distance increased in both groups [RBF group: Δ6MWD = +23.63 (30.70) m, p = 0.002** vs. control group: Δ6MWD = +36.21 (43.54) m, p = 0.0005**].
There was no statistically significant difference in the change of cardiopulmonary exercise capacity as assessed by the 6MWD between the RBF group (left) and the control group (right) after the 4 weeks of pulmonary rehabilitation. Within both groups, there was a statistically significant improvement in 6MWD after pulmonary rehabilitation. ** = highly significant.
There was no statistically significant difference in the change of cardiopulmonary exercise capacity as assessed by the 6MWD between the RBF group (left) and the control group (right) after the 4 weeks of pulmonary rehabilitation. Within both groups, there was a statistically significant improvement in 6MWD after pulmonary rehabilitation. ** = highly significant.
No significant differences were found in the quality of life between the two groups (fig. 3). Within-group comparisons for both study groups showed highly significant improvements in health-related quality of life after 4 weeks of pulmonary rehabilitation [RBF group: ΔCRQ score = +0.64 (0.85), p < 0.001** vs. control group: ΔCRQ score = +0.48 (0.85), p < 0.001**].
There was no statistically significant difference in the change of health-related quality of life as assessed by the CRQ between the RBF group (left) and the control group (right). A statistically significant improvement in health-related quality of life was observed in both groups after 4 weeks. ** = highly significant.
There was no statistically significant difference in the change of health-related quality of life as assessed by the CRQ between the RBF group (left) and the control group (right). A statistically significant improvement in health-related quality of life was observed in both groups after 4 weeks. ** = highly significant.
Changes in Cardiac Autonomic Function
Between-group statistical comparisons of improvements in time and frequency domain measures of parasympathetic-induced HRV are shown in table 3. No significant differences between changes in the mean values of R-R interval and R-R interval variability were found between both groups.
Within-group comparisons analyses showed improvements in the measurements of parasympathetic-induced HRV in both time and frequency domains (table 3; fig. 4). However, compared to baseline, no significant differences in any measurement of parasympathetic-induced HRV in frequency domains were found over the 4 weeks of pulmonary rehabilitation in the control group. Only the RBF group showed significant improvements in resting SDNN (ms) over the 4 weeks of pulmonary rehabilitation; SDNN increased from 32.0 (23.7) ms to 48.0 (52.8) ms (p = 0.037*).
There was no statistically significant difference in the change of cardiac autonomic function as assessed by rMSSD between the RBF group (left) and the control group (right).
There was no statistically significant difference in the change of cardiac autonomic function as assessed by rMSSD between the RBF group (left) and the control group (right).
Discussion
This is the first RCT comparing the effects of a 4-week rehabilitation program including controlled breathing using respiratory biofeedback to a 4-week rehabilitation program alone on pulmonary function (pulmonary function tests), cardiopulmonary exercise capacity, health-related quality of life and cardiac autonomic modulation. The initial hypothesis is not corroborated by the present observations; controlled breathing using a technique of RBF does not produce additional benefits compared to conventional pulmonary rehabilitation programs.
Pulmonary Function, Health-Related Quality of Life and Cardiopulmonary Exercise Capacity
It has been established that pulmonary rehabilitation in patients with COPD results in increased exercise tolerance, peripheral muscle force and health-related quality of life without any effects on pulmonary function or arterial blood gas levels [40,41,42,43]. Accordingly, we found significant improvements in both health-related quality of live (Chronic Respiratory Questionnaire) and exercise tolerance (6MWD) but not on any pulmonary function parameters reflecting airflow limitation, inspiratory lung capacity or lung hyperinflation over the 4 weeks of pulmonary rehabilitation.
Reviewing of the literature on controlled breathing techniques, such as DB, PLB and prolonged exhalation in patients with COPD, reveals that the use of each of these modalities has certain clinical benefits: PLB appears to be an effective way to decrease dyspnoea [12,13], improve the walking distance in the 6MWT [13] and improve gas exchange [15,44]. Furthermore, PLB has been shown to provide sustained improvement in exertional dyspnoea and physical performance [45]. DB in patients with COPD is associated with improvement of blood gases at the expense of a greater inspiratory muscle loading [16,23,46]. Several studies have demonstrated that COPD patients are able to voluntarily change their breathing pattern to more abdominal movement and less thoracic excursion [24,47,48]; however, no changes in ventilation distribution could be observed [48].
However, contradictory evidence exists about the effectiveness of controlled breathing techniques: Garrod [49] found that pursed lips breathing did not improve walking distance in nonspontaneous pursed-lips breathing COPD patients and Gosselink et al. [24] found that DB was associated with a decrease in mechanical efficiency in comparison with natural breathing in patients with COPD. Further analysis of the literature to date does not support the use of DB to improve ventilation, gas exchange, or the work of breathing in patients with COPD [57,58,59]. In summary, DB seems to have negative and positive effects, but the latter appears to be caused by simply slowing the respiratory rate [15].
To date, no trials have been published that investigated patients’ ability to train these breathing techniques all together and evaluate their overall effects over a prolonged period of time. In this study, no additional benefits from controlled breathing interventions in pulmonary function, health-related quality of life and cardiopulmonary exercise capacity were observed over the 4 weeks of pulmonary rehabilitation.
Cardiac Autonomic Function
Giardino et al. [22] found that after 10 weeks of controlled breathing, patients with COPD showed statistically and clinically significant improvements in parasympathetic tone [22]. These results suggest that breathing interventions per se can produce long-term changes in multiple organ systems that are affected by autonomic control. Although the improvement in parasympathetic-induced HRV in time domain of the RBF group in this study was significant, there seems to be no significant additional benefit from controlled breathing on parasympathetic tone over the 4 weeks of pulmonary rehabilitation.
Previous studies have established that patients with COPD have predominant sympathetic tone at rest, as assessed by increased resting heart rate and reduced HRV [50], and that the presence of cardiac autonomic dysfunction is generally associated with worse prognosis [51,52,53]. Predominant resting sympathetic tone in COPD may even have negative consequences on inflammation, cachexia and skeletal muscle dysfunction [54]. In accordance with this, we found that mean resting HR was elevated [89.1 (20.7) beats/min] and HRV was reduced [34.50 (31.10) ms] compared to published control data [55,56], underlining increased sympathetic tone at rest in the patients of this study.
HF power, SDNN and rMSSD are widely accepted to reflect the degree of parasympathetic induced modulation of heart rate [33,37,38]. Interestingly we found that, after 4 weeks of intensive cardio-pulmonary exercise training, all variables reflecting parasympathetic tone increased. The increase in SDNN of the RBF group was statistically significant. These results show that cardiac autonomic dysfunction can be positively influenced by intensive physical exercise training in patients with COPD. However, the pathophysiological mechanism underlying the substantially enhanced resting parasympathetic tone due to cardiopulmonary exercise training in patients with COPD remains unclear.
Limitations of the Study
It should be stressed that a number of patients found it difficult to change their breathing pattern during exercise performance. The controlled breathing techniques used in this study might be more beneficial if trained separately. It could be possible that pursed-lips breathing might be counteracted by those which might increase the work of breathing, such as deep DB [24]. In addition, a number of patients found it difficult to change their breathing pattern during exercise performance and thus the controlled breathing techniques used in this study may be more effective if trained separately rather than in combination. Furthermore, we cannot exclude that controlled breathing has some long-term effects. As rehabilitation is a relatively powerful intervention, it is difficult to secure improvements over and above those observed by rehabilitation alone. Some clinically relevant parameters were not assessed, that is, tidal volume, inspiration time/expiration time ratio and dynamic hyperinflation during exercise; thus we cannot exclude that controlled breathing has a positive effect on hyperinflation. Future studies should assess the effects of controlled breathing on dynamic hyperinflation and assess the long-term effects of controlled breathing. Long-term effects of controlled breathing might be helpful to develop appropriate therapeutic strategies and to improve long-term therapeutic management in COPD.
The number of patients included in our study was relatively small (n = 40). However, the 95% confidence intervals of the difference in ΔFEV1 between the two groups were –100 to 80 ml, thus we have excluded a significantly larger ΔFEV1 in the RBF group of more than 80 ml which is within the range of the minimal clinical importance. Similarly, the 95% confidence intervals of the difference in Δ6MWD between the two groups were –37 to 12 m, thus we have excluded a significantly larger Δ6MWD in the RBF group of more than 12 m, again well within the range of the minimal clinical importance.
Conclusions
The initial hypothesis is not corroborated by the present observations that controlled breathing sessions using a technique of RBF has any influence on pulmonary function, cardiopulmonary exercise capacity, health-related quality of life and cardiac autonomic function. The improvements on resting HRV observed in both groups show that cardiac autonomic dysfunction can be positively influenced by 4 weeks of intensive physical exercise training in patients with COPD.
Financial Disclosure and Conflicts of Interest
None of the authors has a conflict of interest related to the content of the manuscript.
References
ClinicalTrials.gov: Effects of breathing retraining in patients with chronic obstructive pulmonary disease (COPD). ClinicalTrials.gov identifier: NCT01175265.