Abstract
The analysis of the distribution of the calbindin-D28k and calretinin immunoreactive (CBir and CRir) systems recently described in the brain of anuran and urodele amphibians was very useful for the interpretation of many otherwise indistinct brain regions and cell masses. In the present study we have followed a similar approach to investigate the distribution of CBir and CRir cell bodies and fibers in the brain of Dermophis mexicanus, a member of the much neglected third amphibian order of gymnophionans. The pattern of distribution obtained showed particular characteristics in Dermophis, such as the existence of abundant CRir elements in the olfactory bulbs and CBir and CRir cell populations in pallial areas. The distinct distribution of the two proteins allowed the tentative identification of currently described subregions, mainly in the amygdaloid complex and hypothalamic areas. The analysis of the diencephalon and brainstem distribution framed in the neuromeric model highlighted common traits with other amphibians but also specific features. Therefore, the immunohistochemical detection of calcium-binding proteins has served to discern cell populations and has helped to demonstrate neuronal heterogeneity. However, it should be pointed out that a straightforward comparison based only on the presence of these proteins should not be made due to the great variability observed in well-established homologous regions in the brain of different vertebrates, as evidenced within the class Amphibia.

Introduction
Extant amphibians comprise 3 lineages – salamanders (Urodela or Caudata), frogs and toads (Anura), and caecilians (Gymnophiona, Apoda, or Caecilia) – which contain more than 6,000 species. The little known order Gymnophiona comprises 171 species of circumtropical distribution [Frost, 2007]. They are limbless, burrowing animals with worm-like bodies that move like snakes and posses a sense organ unique among vertebrates, i.e. the tentacle, which is probably involved in tactile and chemoreceptive functions, and their visual system was regarded as nonfunctional or degenerated [Engelhardt, 1924; Noble, 1931; Taylor, 1968; Storch and Welsch, 1973; Nussbaum and Wilkinson, 1989; Duellman and Trueb, 1994; Himstedt, 1996]. Interestingly, morphology- and molecule-based studies have disagreed profoundly regarding extant amphibian relationships. Most morphological and paleontological studies of living and fossil amphibians support the hypothesis that salamanders and frogs are sister lineages (the Batrachia hypothesis) and that caecilians are more distantly related [Milner, 1988; Trueb and Cloutier, 1991; Duellman and Trueb, 1994]. In contrast, interpretations of molecular data based on nuclear and mitochondrial rRNA sequences suggested that salamanders and caecilians are sister groups, with the exclusion of frogs [Larson and Wilson, 1989; Hedges et al., 1990, 1993; Bolt, 1991; Hay et al., 1995; Feller and Hedges, 1998]. However, subsequent comparative studies of the complete mitochondrial genomes of several gymnophionans supported the monophyly of living amphibians with respect to other living groups of tetrapods, and a sister group relationship of salamanders and frogs [Zardoya and Meyer, 2001; San Mauro et al., 2004].
In contrast to the relatively well-investigated neuroanatomy of anurans and urodeles, the gymnophionans have received little attention in the neuroscientific literature, not least because of the difficulty to breed them in the laboratory, thereby restricting the number of test animals. The brain development of frogs and salamanders differs considerably. The brains of frogs are morphologically much more complex than those of salamanders [Roth et al., 1993; ten Donkelaar, 1998a, b]. On the other hand, salamanders and caecilians show similarities in many features of brain development [Roth et al., 1993]. In both orders, the identification of individual nuclei in the brain is arduous since cell segregation is hardly present in any brain region. In addition, major structures, such as the cerebellum, are absent or currently unrecognized in the gymnophionan brain [Kühlenbeck, 1922, 1975, 1978]. These shared patterns would seemingly contradict the Batrachia hypothesis. However, the simple brain morphology of salamanders may be a secondarily derived condition associated with an increase in genome, cell size, and paedomorphic evolution [Roth et al., 1993, 1997]. In fact, the available experimental studies conducted in the brain of gymnophionans have shown many complex features of its organization that could not be detected on the basis of cytoarchitecture alone and that were distinct from those of anurans and urodeles. These features of gymnophionans include, among others, the presence of particular cholinergic and somatostatinergic cell groups in the thalamus, the presence of a well-developed mesencephalic group of dopaminergic cells, the existence of complex cholinergic and nitrergic cell populations in the basal ganglia, or the particular arrangement of the motor nuclei in the brainstem [González and Smeets, 1994, 1997; Pinelli et al., 1997; Hilscher-Conklin et al., 1998; Ebersole and Boyd, 2000; Ebersole et al., 2001; Sánchez-Camacho et al., 2001; González et al., 2002a, b; López et al., 2006, 2007]. Therefore, any study that attempts to clarify the amphibian condition of a given system must include results not only of anurans and urodeles but also of gymnophionans.
Immunohistochemical detection of calcium-binding proteins (CBPs) in the brain has been demonstrated to be a useful tool for distinguishing subpopulations of neurons. Among the CBPs, calbindin-D28k (CB) and calretinin (CR) abundantly occur in various types of neurons in the central nervous system of mammals [García-Segura et al., 1984; Fournet et al., 1986; Jones and Hendry, 1989; Celio, 1990; Jacobowitz and Winsky, 1991; Baimbridge et al., 1992; Résibois and Rogers, 1992; Rogers and Résibois, 1992; Winsky et al., 1992; Andressen et al., 1993; Arai et al., 1994; De Biasi et al., 1994; Molinari et al., 1994; Gutiérrez et al., 1995]. These proteins act as fast calcium buffers and actively operate in calcium-mediated signal transduction [Heizmann and Braun, 1992; Polans et al., 1996]. However, the dissimilarities in CB and CR sequence [Parmentier, 1990] reflect functional and evolutionary differences between the two proteins in terms of adaptation to different targets [Schwaller et al., 2002; Palczewska et al., 2003]. CR is considered a pure Ca2+ buffer which acts as passive modulator of the cytosolic calcium levels [Schmidt et al., 2007], whereas CB acts as a sensor that regulates the degradation of inositol messengers in an activity-dependent manner [Schmidt et al., 2005] and has a neuroprotective capability to prevent degeneration [Wang et al., 2008]. Thus, each protein seems to play specific roles in different neuron subsets. Moreover, the frequent use of CBPs in anatomical studies is mainly based on their occurrence in defined neuronal classes and, within the cell, usually throughout the whole cytoplasm including that of fine processes, which results in Golgi-like staining by immunohistochemistry. In addition, similar studies in nonmammalian vertebrates corroborated that the localization of CB and CR is extremely useful for identifying nuclear boundaries that are difficult to distinguish based on cytoarchitectonic criteria alone [Lunam, 1989; Rodríguez-Moldes et al., 1990a, b; Pombal and Puelles, 1999; Dávila et al., 2000; Díaz-Regueira and Anadón, 2000; Huesa et al., 2006; Morona et al., 2006a, b, 2007a].
Previous studies in anurans and urodeles demonstrated that CB and CR are powerful markers of well-segregated positive neuronal populations, fiber tracts, and neuropils in the brains of amphibians [Milán and Puelles, 2000; Morona and González, 2008, 2009]. Moreover, the patterns observed were consistent with the conceptual subdivision entities contemplated in the segmental paradigm of the brain, and the analysis of the results attending to this paradigm highlighted comparisons across species of different vertebrate classes [Pombal and Puelles, 1999; Dávila et al., 2000; González et al., 2002; Puelles and Rubenstein, 2003; Morona and González, 2008, 2009]. Our main aim in the present study was to provide a comprehensive description of the distribution patterns of neurons and fibers which are CB and CR immunoreactive (CBir and CRir, respectively) throughout the full extent of the brain of one gymnophionan amphibian, Dermophis mexicanus, with a view to identifying subpopulations of neurons not distinguished on the basis of cytoarchitecture alone. This study, in comparison with previous studies in anurans and urodeles [Milán and Puelles, 2000; Morona and González, 2008, 2009], will help to gain a better understanding of the anatomical complexity of the amphibian brain. The current neuromeric models [Gilland and Baker, 1993; Marín and Puelles, 1995; Fritzsch, 1998; Cambronero and Puelles, 2000; Díaz et al., 2000; Puelles and Rubenstein, 2003; Straka et al., 2006] are used as a framework for the interpretation of the results, thus allowing a ready comparison among amphibians and other vertebrates.
Materials and Methods
Animals and Tissue Processing
For the present study, 6 specimens of the gymnophionan D. mexicanus were used. Three adults (body length about 50 cm) were males and 1 was a female (about the same size), but the other 2 brains corresponded to adult specimens whose sex was not determined. The animals were adults obtained from authorized commercial suppliers (Triton Madrid, Spain). They were kept in a room with a controlled temperature (25°C) and natural light/dark conditions for a few days before being used. The original research reported herein was performed according to the regulations and laws of the European Union (86/609/EEC) and Spain (Royal Decree 1201/2005) for the care and handling of animals in research.
The animals were anesthetized in a 0.3% solution of tricaine methanesulfonate (MS222, pH 7.4; Sigma, St. Louis, Mo., USA) and perfused transcardially with saline followed by 200 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). The brains were removed and kept in the same fixative for 2–3 h. Subsequently, they were immersed in a solution of 30% sucrose in PB for 5 h at 4°C until they sank, embedded in a solution of 20% gelatin with 30% sucrose in PB, and then immersed in a 3.7% formaldehyde solution at 4°C for 8–10 h. The brains were cut on a freezing microtome at 40 µm in the transverse or sagittal plane and collected in cold PB.
CB and CR Immunohistochemistry
The free-floating sections were rinsed twice in PB, treated with 1% H2O2 in PB for 15 min to reduce endogenous peroxidase activity, rinsed again 3 times in PB, and processed via the peroxidase antiperoxidase method [Sternberger, 1979]. This included a first incubation of the sections in a mouse anti-CB or rabbit anti-CR serum (catalog No. 300 and 7699/4, respectively; Swant, Bellinzona, Switzerland), diluted 1:1,000 in PB containing 0.5% Triton X-100 (PBS-T), for 48–72 h at 4°C. Subsequently, they were rinsed in PB for 10 min and incubated in the secondary antiserum goat anti-mouse (diluted 1:50 in PBS-T; Dako, Glostrup, Denmark) or swine anti-rabbit (diluted 1:50 in PBS-T; Dako) for 60 min at room temperature. After rinsing, the sections were incubated for 90 min in either mouse or rabbit peroxidase antiperoxidase complex (diluted 1:500 in PBS-T; Dako) and rinsed 3 times in PB. Finally, the sections were stained in 0.5 mg/ml 3,3′diaminobenzidine (DAB; Sigma) or in DAB intensified with nickel [Adams, 1981] with 0.01% H2O2 in PB for 10–20 min. Some series of sections were stained according to the glucose oxidase method [Shu et al., 1988], which enhances the staining of fibers and terminals. The sections were then mounted on glass slides from a solution of 0.25% gelatin in 0.05 M Tris-HCl buffer (pH 7.6), and after dehydration the slides were coverslipped with Entellan (Merck, Darmstadt, Germany). Some sections were counterstained with cresyl violet to facilitate analysis of the results.
Double Immunohistofluorescence
To study the colocalization/codistribution of CB and CR, a 2-step protocol for immunohistofluorescence was used with antibody cocktails as follows: (1) incubation for 72 h at 4°C in a mixture of primary mouse anti-CB antibody and rabbit anti-CR antiserum (both diluted 1:1,000 in PBS-T) and (2) a second incubation for 90 min at room temperature in a mixture of Alexa 488-conjugated goat anti-mouse (green fluorescence, diluted 1:300 in PBS-T; Molecular Probes, Eugene, Oreg., USA) and Alexa 594-conjugated goat anti-rabbit (red fluorescence, diluted 1:500 in PBS-T; Molecular Probes). After rinsing, the sections were mounted on glass slides and coverslipped with Vectashield (Vector, Burlingame, Calif., USA).
Controls and Specificity of the Antibodies
Prior to all incubations in the second antibody cocktails, the sections were incubated for 1 h at room temperature in normal serum of the species in which the secondary antibodies were obtained. Immunohistochemical control experiments involved parallel incubation of alternate sections with antiserum raised against different antigens, with normal serum, or with the omission of primary antiserum. No residual immunostaining was detected.
The specificity of the antibodies used against CB and CR was assessed by the commercial company (Swant). The monoclonal antibody anti-CB used in this study is a mouse IgG produced by hybridization of mouse myeloma cells with spleen cells from mice immunized with CB purified from chicken gut. Although the actual CB molecule in Dermophis has not been characterized, the calb-1 gene coding this protein in Xenopus (gene ID 399307) is highly conserved in humans, chimpanzees, dogs, cows, mice rats, chickens, and zebrafish. The sequence of Xenopus shows 97% similarity with that of the chick and includes the EF domain. Furthermore, the anti-CB used has been tested via Western blot with brain extracts of several species of amphibians and labeled a single band of the expected molecular weight (28 kDa) that corresponds well with a similar band labeled in the lane of rat brain extract [Morona and González, 2008]. Additionally, because CB is highly homologous to CR (60% of coincidence in the primary amino acid sequence) [Rogers, 1987], we evaluated the lack of cross-reactivity by means of control experiments [Morona et al., 2006a, b, 2007a, b] in which sections were incubated in the anti-CB serum preabsorbed at 4°C overnight with CR protein (1 µg/1 ml of the diluted antibody; Swant). Control sections were then processed in the same manner as those incubated with the unabsorbed antisera. As a result, no difference in the staining pattern of the antibody was observed, except for the overall staining, which was weaker than under normal conditions. The lack of cross-reactivity between the CB and the CR antibodies or antiserum in the present study was clear in that many cells were distinctly stained for either CB or CR and only a small portion of neurons was doubly labeled in the combined experiments.
The antiserum against CR was produced in rabbits by immunization with recombinant human CR. Its specificity has been demonstrated for many vertebrate species, and it does not cross-react with CB. The CR protein in amphibians (predicted from locus XP 002931730 in Xenopus) has 82% similarity with the human CR. In Dermophis, anti-CR was preabsorbed with 1 µg/ml antigen isolated from rat brain at 4°C overnight, after which immunostaining was completely abolished. At the same time, preabsorption of the anti-CR antibody under the same conditions with 100 µg/ml CB (from chicken gut) did not affect staining. Furthermore, the primary antibodies used in our study have previously been employed in numerous amphibian species [Gábriel et al., 1998; Marín et al., 1998; Necchi et al., 1999; Uray and Gona, 1999; Edmonds et al., 2000; Milán and Puelles, 2000; Brox et al., 2003; Morona and González, 2008, 2009]. The same anti-CR has been tested via Western blot with brain extracts of several species of amphibians and labeled a single band of the expected molecular weight (29 kDa) that corresponds well with a similar band labeled in the lane of rat brain extract [Morona and González, 2008].
Evaluation and Presentation of the Results
The localization of CBir and CRir cell bodies and fibers was studied throughout the brain in both the single-labeled sections and the double-labeled sections. Their relative localization was framed within the newly defined territories in the amphibian telencephalon and the segmental model proposed for the caudal prosencephalon and brainstem (fig. 1) recently adapted for anurans and urodeles [Morona and González, 2008, 2009]. The distribution of CB- and CR-labeled structures was charted by means of a camera lucida in a series of transverse sections from rostral to caudal (fig. 2, 3). The chartings are meant to indicate the relative positions and densities of immunolabeled structures in the brain. Selected single-labeled transverse (fig. 4, 5, 6, 7) or sagittal (fig. 8, 9) sections were photographed with a digital camera (Coolpix 930; Nikon). The double-labeled sections were analyzed with an Olympus BX51 microscope equipped for fluorescence with appropriate filter combinations, and selected sections were photographed (fig. 10) with a digital camera (Olympus DP70). In all cases, the contrast and brightness of the photomicrographs were adjusted using Adobe Photoshop 7.0 (Adobe Systems, San Jose, Calif., USA).
Scheme of a lateral view of the brain of D. mexicanus in which the revised and simplified prosomeric model has been adapted [Puelles and Rubenstein, 2003]. There are 3 segments in the diencephalon, i.e. prosomeres 1–3. The rostralmost forebrain contains the basal and alar parts of the hypothalamus. Following this model, the hypothalamus is divided into rostral and caudal transverse subdomains. It has also been subdivided into separate areas in the alar and basal parts: the preoptic (PO), SPV, suprachiasmatic (SC), paraventricular (PV), tuberal (Tub), and mammillary (Ma) regions. The telencephalon represents an extension of the alar hypothalamic region and is further subdivided into the pallium and subpallium. The segmentation of the brainstem follows that described previously [González et al., 2002a]. The a–z and a–w levels of the sections shown in figures 2 and 3 are indicated.
Scheme of a lateral view of the brain of D. mexicanus in which the revised and simplified prosomeric model has been adapted [Puelles and Rubenstein, 2003]. There are 3 segments in the diencephalon, i.e. prosomeres 1–3. The rostralmost forebrain contains the basal and alar parts of the hypothalamus. Following this model, the hypothalamus is divided into rostral and caudal transverse subdomains. It has also been subdivided into separate areas in the alar and basal parts: the preoptic (PO), SPV, suprachiasmatic (SC), paraventricular (PV), tuberal (Tub), and mammillary (Ma) regions. The telencephalon represents an extension of the alar hypothalamic region and is further subdivided into the pallium and subpallium. The segmentation of the brainstem follows that described previously [González et al., 2002a]. The a–z and a–w levels of the sections shown in figures 2 and 3 are indicated.
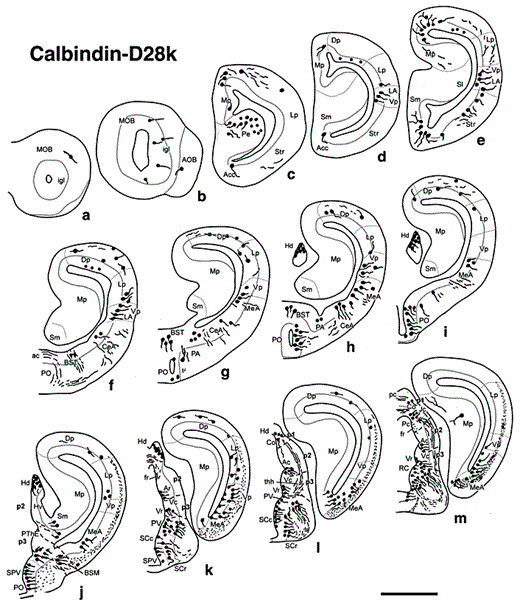
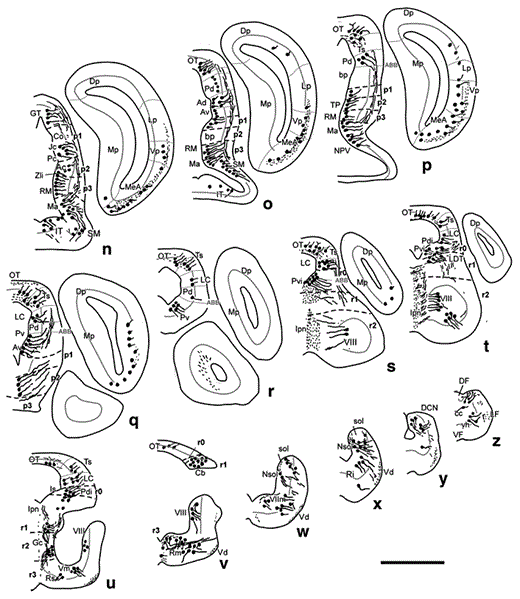
Diagrams of transverse sections of the brain of D. mexicanus (at the rostrocaudal levels indicated in fig. 1) showing the distribution of CBir cell bodies (large dots) and fibers (small dots, wavy lines) in the right half of each section. Scale bar = 1,000 µm.
Diagrams of transverse sections of the brain of D. mexicanus (at the rostrocaudal levels indicated in fig. 1) showing the distribution of CBir cell bodies (large dots) and fibers (small dots, wavy lines) in the right half of each section. Scale bar = 1,000 µm.
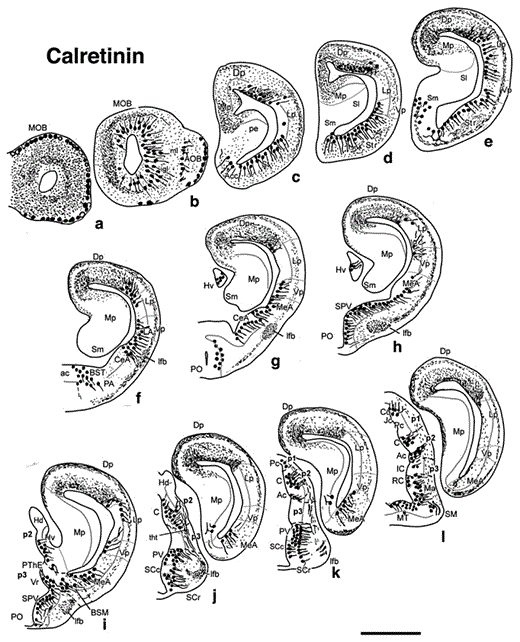
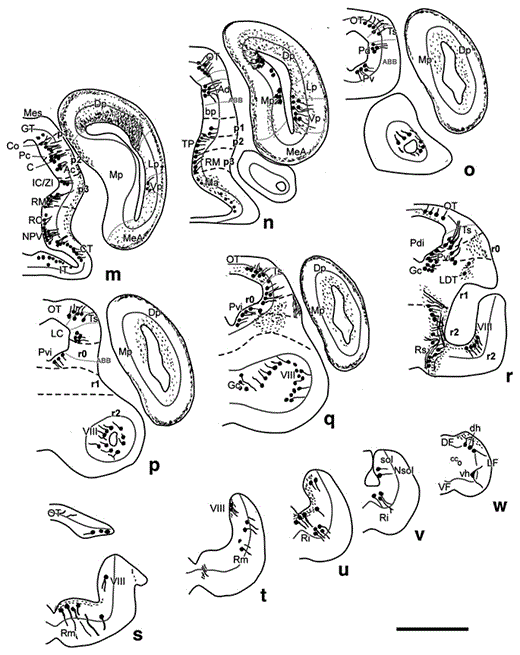
Diagrams of transverse sections of the brain of D. mexicanus (at the rostrocaudal levels indicated in fig. 1) showing the distribution of CRir cell bodies (large dots) and fibers (small dots, wavy lines) in the right half of each section. Scale bar = 1,000 µm.
Diagrams of transverse sections of the brain of D. mexicanus (at the rostrocaudal levels indicated in fig. 1) showing the distribution of CRir cell bodies (large dots) and fibers (small dots, wavy lines) in the right half of each section. Scale bar = 1,000 µm.
Photomicrographs of singly stained transverse sections showing CBir and CRir cells and fibers (indicated in each photograph) in prosencephalic areas of D. mexicanus.a AOB. b MOB. c Detail of CBir cells in the postolfactory eminence. d, e Detail of CB and CR distribution in the dorsal pallium at comparable levels in the rostral telencephalic hemisphere. f CRir cells and fibers in the rostral striatum. g Detail of the caudal CBir cells in the medial amygdala. h CR immunoreactivity in the caudal telencephalic areas, just rostral to the anterior commissure. i, j CBir and CRir cells and fibers in diencephalic and hypothalamic areas at comparable levels. k, l Detail of CBir cells in the mammillary region and CRir cells in the tuberal region, respectively. m CBir cells and fibers in the dorsal nucleus of the habenula. n CRir cells and fibers in the ventral habenular nucleus. o CRir cells at caudal diencephalic levels in the pretectal and thalamic regions. Scale bars = 100 (a–j, m–o) and 50 µm (k, l).
Photomicrographs of singly stained transverse sections showing CBir and CRir cells and fibers (indicated in each photograph) in prosencephalic areas of D. mexicanus.a AOB. b MOB. c Detail of CBir cells in the postolfactory eminence. d, e Detail of CB and CR distribution in the dorsal pallium at comparable levels in the rostral telencephalic hemisphere. f CRir cells and fibers in the rostral striatum. g Detail of the caudal CBir cells in the medial amygdala. h CR immunoreactivity in the caudal telencephalic areas, just rostral to the anterior commissure. i, j CBir and CRir cells and fibers in diencephalic and hypothalamic areas at comparable levels. k, l Detail of CBir cells in the mammillary region and CRir cells in the tuberal region, respectively. m CBir cells and fibers in the dorsal nucleus of the habenula. n CRir cells and fibers in the ventral habenular nucleus. o CRir cells at caudal diencephalic levels in the pretectal and thalamic regions. Scale bars = 100 (a–j, m–o) and 50 µm (k, l).
Photomicrographs of singly stained transverse sections showing CBir and CRir cells and fibers (indicated in each photograph) in diencephalic and hypothalamic areas of D. mexicanus.a CR-stained section through the diencephalon and hypothalamus highlighting the main prosomeric boundaries. b CB-stained section caudal to that shown in a. c CR immunoreactivity marks p2 and the dorsal pallium intensely in this section slightly rostral to that shown in b. d Detail of CRir cells in pretectal and caudal thalamic areas. e Distribution of CBir cells and fibers in a classical ‘transverse’ section through mesencephalic areas, the basal diencephalon, and the hypothalamus. Scale bars = 100 µm.
Photomicrographs of singly stained transverse sections showing CBir and CRir cells and fibers (indicated in each photograph) in diencephalic and hypothalamic areas of D. mexicanus.a CR-stained section through the diencephalon and hypothalamus highlighting the main prosomeric boundaries. b CB-stained section caudal to that shown in a. c CR immunoreactivity marks p2 and the dorsal pallium intensely in this section slightly rostral to that shown in b. d Detail of CRir cells in pretectal and caudal thalamic areas. e Distribution of CBir cells and fibers in a classical ‘transverse’ section through mesencephalic areas, the basal diencephalon, and the hypothalamus. Scale bars = 100 µm.
Photomicrographs of singly stained transverse sections showing CBir and CRir cells and fibers (indicated in each photograph) in mesencephalic, isthmic, and rhombencephalic areas of D. mexicanus.a CBir cells are shown in the medial part of the OT, in the reduced torus semicircularis and the laterocaudal nucleus in the mesencephalon, and in the posterodorsal and posteroventral isthmic nuclei. b Detail of CRir cells at caudal levels in the OT. c Detail of CRir cells in the posteroventral isthmic nucleus. d Section immunoreacted for CB, caudal to that shown in a, that considering the brain flexure corresponds to more dorsal levels (see fig. 1) and shows the distinct labeling in the tectal and isthmic regions. e, f Transverse sections passing through the caudal mesencephalon and the upper rhombencephalon that correspond with actual horizontal sections and show the CBir cells and fibers (e) and a detail of the conspicuous CRir cell population in the Gc (f). Scale bars = 100 µm.
Photomicrographs of singly stained transverse sections showing CBir and CRir cells and fibers (indicated in each photograph) in mesencephalic, isthmic, and rhombencephalic areas of D. mexicanus.a CBir cells are shown in the medial part of the OT, in the reduced torus semicircularis and the laterocaudal nucleus in the mesencephalon, and in the posterodorsal and posteroventral isthmic nuclei. b Detail of CRir cells at caudal levels in the OT. c Detail of CRir cells in the posteroventral isthmic nucleus. d Section immunoreacted for CB, caudal to that shown in a, that considering the brain flexure corresponds to more dorsal levels (see fig. 1) and shows the distinct labeling in the tectal and isthmic regions. e, f Transverse sections passing through the caudal mesencephalon and the upper rhombencephalon that correspond with actual horizontal sections and show the CBir cells and fibers (e) and a detail of the conspicuous CRir cell population in the Gc (f). Scale bars = 100 µm.
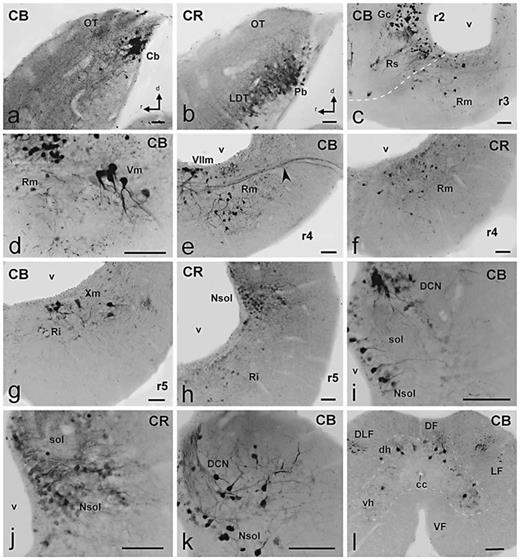
Photomicrographs of singly stained sagittal (a, b) or transverse (c–l) sections showing CBir and CRir cells and fibers (indicated in each photograph) in the hindbrain of D. mexicanus.a, b Comparable sagittal sections showing the dorsal and lateral rim of the isthmo-mesencephalic boundary. CB intensely stained some cells in a complementary pattern to that of CR. c Distribution of numerous intensely CBir cells in the Gc and superior reticular nucleus in r2 and the reticular median nucleus in r3. d Detail of large CBir cells in the region of the trigeminal motor nucleus in r3. e, f Labeled reticular cells for each protein in r4 (arrowhead in e points to labeled fibers in the facial nerve). g Section at caudal levels of the rhombencephalon showing CBir in some large cells in the region of the vagal motor nucleus and in the inferior reticular nucleus. h CR staining in the basal reticular cells and in the alar group of the nucleus of the solitary tract. i, j Detail of CBir and CRir cells in the caudal dorsal alar region showing distinct labeling in the nucleus of the solitary tract and the dorsal column nucleus. k Pattern of CB staining close to the obex in the nucleus of the solitary tract and the dorsal column nucleus. l Distribution of CBir cells in the first segments of the spinal cord. Scale bars = 100 µm.
Photomicrographs of singly stained sagittal (a, b) or transverse (c–l) sections showing CBir and CRir cells and fibers (indicated in each photograph) in the hindbrain of D. mexicanus.a, b Comparable sagittal sections showing the dorsal and lateral rim of the isthmo-mesencephalic boundary. CB intensely stained some cells in a complementary pattern to that of CR. c Distribution of numerous intensely CBir cells in the Gc and superior reticular nucleus in r2 and the reticular median nucleus in r3. d Detail of large CBir cells in the region of the trigeminal motor nucleus in r3. e, f Labeled reticular cells for each protein in r4 (arrowhead in e points to labeled fibers in the facial nerve). g Section at caudal levels of the rhombencephalon showing CBir in some large cells in the region of the vagal motor nucleus and in the inferior reticular nucleus. h CR staining in the basal reticular cells and in the alar group of the nucleus of the solitary tract. i, j Detail of CBir and CRir cells in the caudal dorsal alar region showing distinct labeling in the nucleus of the solitary tract and the dorsal column nucleus. k Pattern of CB staining close to the obex in the nucleus of the solitary tract and the dorsal column nucleus. l Distribution of CBir cells in the first segments of the spinal cord. Scale bars = 100 µm.
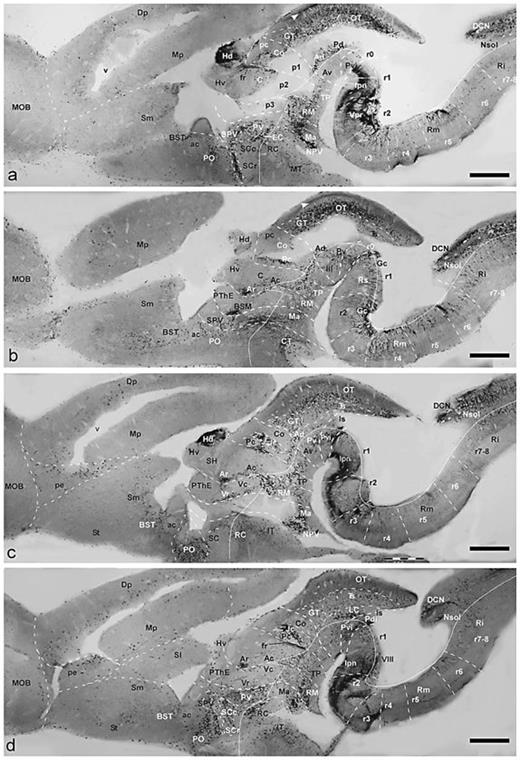
Parasagittal sections showing CBir cells and fibers in the brain of D. mexicanus, presented in lateromedial order (a–d). Dorsal is oriented upward and rostral to the left. The diencephalic prosomeres p1–p3, the isthmus and rhombomeres are delimited by dash lines. The alar-basal boundary is indicated by a fine line. Scale bars = 1 mm.
Parasagittal sections showing CBir cells and fibers in the brain of D. mexicanus, presented in lateromedial order (a–d). Dorsal is oriented upward and rostral to the left. The diencephalic prosomeres p1–p3, the isthmus and rhombomeres are delimited by dash lines. The alar-basal boundary is indicated by a fine line. Scale bars = 1 mm.
Parasagittal sections showing CRir cells and fibers in the brain of D. mexicanus, presented in lateromedial order (a–d). Dorsal is oriented upward and rostral to the left. The diencephalic prosomeres p1–p3, the isthmus and rhombomeres are delimited by dashed lines. The alar-basal boundary is indicated by a fine line. Scale bars = 1 mm.
Parasagittal sections showing CRir cells and fibers in the brain of D. mexicanus, presented in lateromedial order (a–d). Dorsal is oriented upward and rostral to the left. The diencephalic prosomeres p1–p3, the isthmus and rhombomeres are delimited by dashed lines. The alar-basal boundary is indicated by a fine line. Scale bars = 1 mm.
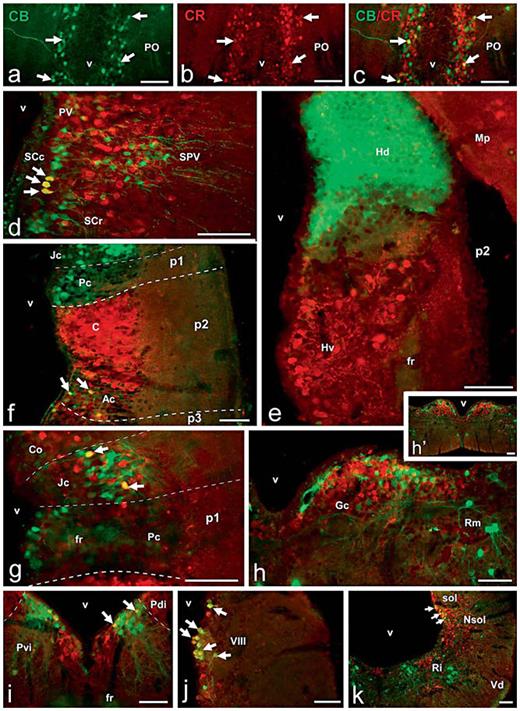
Photomicrographs of singly (a, b) and doubly (c–k) stained transverse sections through the brain of D. mexicanus that illustrates the degree of codistribution/colocalization of both proteins in different regions. a–c Preoptic CBir neurons immunolabeled in green (a) and CRir neurons immunostained in red (b) are widely intermingled, and only a few neurons were double labeled, as observed in yellow in the merged image (arrows in c). d Abundant codistribution of CBir and CRir cells in the hypothalamic areas and actual colocalization only in some cells of the caudal suprachiasmatic nucleus (arrows). e Complementary distribution of CBir and CRir cells and fibers in the habenula. f CB and CR localization in p1 and p2 shows only a restricted colocalization in the anterior nucleus of p2 (arrows). g Codistribution of CBir and CRir cells in the pretectum and actual colocalization only in some cells of the juxtacommissural nucleus. h, h’ Codistribution of CB and CR at the Gc located in rhombomere r2, as observed in low (h’) and high (h) magnifications. i Relative localization of CBir and CRir cells in the isthmic tegmentum and colocalization in Pvi (arrows). j Abundant CB/CR colocalization in cells of the octaval column in the rhombencephalic alar plate. k Simultaneous observation of the distribution of CBir and CRir cells and fibers in the caudal rhombencephalon (arrows point to doubly labeled cells in the nucleus of the solitary tract). Scale bars = 100 µm.
Photomicrographs of singly (a, b) and doubly (c–k) stained transverse sections through the brain of D. mexicanus that illustrates the degree of codistribution/colocalization of both proteins in different regions. a–c Preoptic CBir neurons immunolabeled in green (a) and CRir neurons immunostained in red (b) are widely intermingled, and only a few neurons were double labeled, as observed in yellow in the merged image (arrows in c). d Abundant codistribution of CBir and CRir cells in the hypothalamic areas and actual colocalization only in some cells of the caudal suprachiasmatic nucleus (arrows). e Complementary distribution of CBir and CRir cells and fibers in the habenula. f CB and CR localization in p1 and p2 shows only a restricted colocalization in the anterior nucleus of p2 (arrows). g Codistribution of CBir and CRir cells in the pretectum and actual colocalization only in some cells of the juxtacommissural nucleus. h, h’ Codistribution of CB and CR at the Gc located in rhombomere r2, as observed in low (h’) and high (h) magnifications. i Relative localization of CBir and CRir cells in the isthmic tegmentum and colocalization in Pvi (arrows). j Abundant CB/CR colocalization in cells of the octaval column in the rhombencephalic alar plate. k Simultaneous observation of the distribution of CBir and CRir cells and fibers in the caudal rhombencephalon (arrows point to doubly labeled cells in the nucleus of the solitary tract). Scale bars = 100 µm.
The nomenclature used largely follows that established in similar studies of the anuran and urodeles brains [Milán and Puelles, 2000; Morona and González, 2008, 2009].
Results
The antibodies against CB and CR used in the present study labeled cell bodies and fibers throughout the brain and spinal cord of Dermophis. They revealed distinct patterns of immunohistochemistry for each protein that were constant from animal to animal, and no differences were noted that could suggest sex differences. Mostly segregated patterns for CB and CR were obtained and only in some areas was colocalization of both proteins in some neurons detected by the double-immunohistofluorescence technique.
We describe the distribution of CBir and CRir cells and fibers following the main brain subdivisions from rostral to caudal (fig. 1). The analysis is mainly made on the basis of transverse sections that can be followed in the schemes (fig. 2, 3) and selected sets of microphotographs (fig. 4, 5, 6, 7). However, a specific feature of the gymnophionan brain, not observed in anurans and urodeles, is a pronounced flexure of the longitudinal axis at mesencephalic levels that bends the brainstem in such a manner that the upper rhombencephalon is placed beneath the mesencephalon (see scheme in fig. 1). Thus, conventional ‘transverse’ sections through the diencephalon and rostral brainstem are almost horizontal sections (parallel to the alar/basal boundary). For this reason, the analysis of sagittal sections has been most valuable for identifying the correct position of labeled cell groups and the course of fiber tracts (fig. 8, 9).
We here analyze the distribution of CB and CR using as a framework the distinct components of the telencephalon recently identified in anurans and urodeles, the prosomeric model for the diencephalon and nontelencephalic secondary prosencephalon [Puelles et al., 1996; Milán and Puelles, 2000; Puelles and Rubenstein, 2003], and the neuromeric organization of the brainstem [Straka et al., 1998; Díaz et al., 2000; Straka et al., 2006]. This has been accomplished by direct comparison with the results obtained in anurans and urodeles using the same immunohistochemical techniques [Morona and González, 2008, 2009].
Olfactory Bulbs
The main olfactory bulbs (MOB) of Dermophis are large structures formed in the rostral hemispheres, and conspicuous accessory olfactory bulbs (AOB) are located ventrolaterally to the main bulbs (fig. 1). Both bulbs consist of concentric layers with the incoming olfactory and vomeronasal fibers forming glomeruli in the most superficial layer. In centripetal order, interior to the glomerular layer is the external cellular layer that contains the mitral cells, the extragranular plexiform layer composed of secondary olfactory fibers, the thick internal granular layer, and the ependyma [Northcutt and Kicliter, 1980]. Within the bulbs, CB was only located in scattered cells distributed in the internal granular layer of the MOB and, to a lesser extent, the AOB (fig. 2a, b). In contrast, abundant CRir cells and fibers occupied the olfactory bulbs (fig. 3a, b, 4a, b). An intricate network of labeled fine fibers were localized in practically all layers of the MOB and AOB. The primary olfactory and vomeronasal fibers and the glomeruli that they form in the periphery of the bulbs were intensely CRir. No labeled cells were observed in the periglomerular position. Most CRir cells were found in the internal granular layer of the MOB and AOB and, in the former, those located more peripherally showed a slightly larger size (fig. 4b). Just caudal to the MOB, adjacent to the rostral tip of the medial pallium, a region identified as the postolfactory eminence [Northcutt and Kicliter, 1980] contained a relative large number of CBir small cells (fig. 2c, 4c, 8c, d) and only labeled fibers for CR (fig. 3c, 9c).
Pallium
A peculiarity of the brain of gymnophionans, and not only of Dermophis, is the enormous development of the telencephalic hemispheres and, in particular, the extent of the pallium that is caudally enlarged, laterally covering the diencephalon and the upper brainstem (fig. 2c–t, 3c–q, 8, 9) [Zilles et al., 1981]. The presence of CBir elements in the pallium was observed only in a small population of cells that were concentrated mainly in the medial part of the dorsal pallium and the rostral portion of the medial pallium (fig. 2c–l, 4d, 8). These cells were located in the deep cell layer, which is characteristic of the whole pallium of Dermophis, separated from the ventricular lining (fig. 4d). From mid to caudal telencephalic levels, the number of CBir cells was higher and occupied the ventral aspect of the lateral pallium, an area tentatively identified here as the ventral pallium (see Discussion) (fig. 2e–p). Strikingly different, the amount and intensity of CRir elements in the pallium were very conspicuous (fig. 3c–q, 4e, h, 5c, 9). The pattern of CR labeling varied from rostral to caudal and from medial to lateral pallial regions. As in the case of CB, the large medial pallium was practically devoid of CRir elements, whereas the adjacent dorsal pallium was intensely immunoreactive. At rostral levels of the hemispheres, the dorsal and lateral pallial regions showed a numerous population of intensely CRir cells located close to the ventricular lining and, within the outer thick fiber zone, the deep part was intensely CRir with fine fibers and terminal-like structures, whereas in the superficial part almost no labeling was observed (fig. 3c, d, 4e). In contrast, at caudal levels the superficial fiber zone that covers the dorsal pallium was filled with CRir fibers that formed a band that extended from the external dorsal part of the medial pallium through the dorsal pallium and dispersed in the deep aspect of the lateral and ventral pallial regions and entered the amygdaloid zones (fig. 3e–l, 4h, 5c). Similar to the case of CB, abundant CRir neurons were located in the ventral pallium (described below in Amygdaloid Complex) (fig. 3e–l, 4h).
Septum
Gymnophionans possess a rostral septal area several times the size of the medial pallium, and its rostral tip has been compared with the previously described postolfactory eminence of anurans [Northcutt and Kicliter, 1980]. The septal enlargement at mid telencephalic levels, which has been considered the counterpart of the lateral septal nucleus of other amphibians, is located ventral to the medial pallium and both together form the thick medial wall of the hemisphere (fig. 2e, 3e, 4h). The septal bulge was devoid of CBir and CRir cells and only CRir fibers were located laterally in the septum, close the ventricle (fig. 4h). However, the septal portion that shows no particular enlargement and is located more ventrally has been compared to the medial septal nucleus of anurans and urodeles and showed a reduced population of CRir cells that were located close to the subpial surface (fig. 3e, 9d).
Basal Ganglia
By means of immunohistochemical detection of the dopaminergic innervation of the telencephalon, a striatum proper and a nucleus accumbens were identified as basal ganglia components in the subpallium of gymnophionans [González and Smeets, 1994; González et al., 1994]. The nucleus accumbens was localized at rostral telencephalic levels, ventral to the rostral septal region, and in the present study it was seen to contain only occasional CBir cell bodies and more abundant CRir neurons (fig. 2d, 3c, 4f). Also restricted was the distribution of CBir cells in the large striatum that occupies much of the ventrolateral wall of the hemispheres (fig. 2e). These cells showed pear-shaped somata with a main dendritic process directed toward the pial surface. The population of CRir neurons in the striatum of Dermophis was very large and extended the whole length of the striatal region (fig. 3c–e, 4f). The CRir cells were small neurons and showed dendritic processes mainly oriented ventrolaterally into the striatal neuropil where they arborized (fig. 4f). Most of the striatal neuropil was filled with CRir fibers and terminals that reached this region in the lateral forebrain bundle from diencephalic territories (fig. 3e–k). In Dermophis, no pallidal structures have been previously described. Recently, in urodeles, a pallidal portion in the ventrocaudal telencephalon has been demonstrated in the previously named ventral cellular prominence [Moreno and González, 2007a]. In our study, this area in Dermophis was located ventrally to the central amygdala (see below) and showed abundant intensely CBir cells (fig. 2g, h) and no CRir cells (fig. 3f, 4h).
Amygdaloid Complex
The amygdala of amphibians was traditionally subdivided into pars lateralis and pars medialis on the basis of topography [Northcutt and Kicliter, 1980]. However, research in the last years about the telencephalic organization in anurans and urodeles has proved that at least 3 main amygdaloid subdivisions can be considered that were named for facilitating their comparison with their counterparts in amniotes: lateral, central, and medial [Moreno and González, 2006, 2007a, b]. Several immunohistochemical studies in the brain of gymnophionans support the presence of the same subdivisions of the amygdaloid complex, and we have tentatively identified them in this study [González and Smeets, 1994; González et al., 2002a, b; López et al., 2006, 2007].
The amphibian lateral amygdala is considered a ventral pallial territory located between the lateral pallium (dorsally) and the striatum (ventrally) and is primarily identified by its content in nitrergic cells and fibers, its connections, and the expression of genetic markers [Moreno and González, 2004, 2006]. Within the putative lateral amygdala of Dermophis, a small population of CBir neurons was localized mainly at rostral telencephalic levels (fig. 2d–h). Significantly more abundant were the CRir cells detected in the lateral amygdala that extended more caudally than the CBir cell population (fig. 3d–i, 4h). The central amygdala of amphibians is currently interpreted as a caudal continuation of the striatum; both share the same origin in the developing lateral ganglionic eminence and are distinct in their hodology [Moreno and González, 2005]. In Dermophis, a similar territory occupies most of the previously named pars lateralis of the amygdala [Northcutt and Kicliter, 1980]. In this location, only scarce CBir cells were observed (fig. 2g, h), whereas a large population of CRir neurons formed a caudal continuation of the striatal cell population into the central amygdala (fig. 3f, g). The third component of the amygdaloid complex is the medial amygdala, which is primarily defined by its input from the AOB [Moreno and González, 2003, 2006, 2007a]. In Dermophis, this region would be included in the caudal portion of the former pars lateralis of the amygdala. Its rostral portion caps dorsally the central amygdala and extends caudally in the enlarged caudal pole of the hemispheres (fig. 2g–p, 3g–n). Within the medial amygdala, CBir cells were always detected and were mainly located at its caudal levels (fig. 4g). However, the population of CRir neurons in the medial amygdala outnumbered that of CBir cells and was localized throughout the rostrocaudal extent (fig. 4h, 5c).
In relation to the amygdaloid complex of amphibians, a cell group located medial to the ventral tip of the lateral ventricle at caudal telencephalic levels has been proposed as the bed nucleus of the stria terminalis (BST) [Marín et al., 1998]. This region in anuran amphibians has been characterized by immunohistochemistry, development, and connectivity [Moreno and González, 2006; Moreno et al., 2011]. Its content of CB and CR has been analyzed [Morona and González, 2008] and by strict comparison a similar region has been considered in Dermophis. It was clearly occupied by CBir and CRir cells that formed a column that extended caudally to the anterior commissure (fig. 2g, h, 3f, 4h, 8a–d, 9a–c).
Nontelencephalic Secondary Prosencephalon and Hypothalamus
The set of structures comprised in this part of the brain is localized topologically rostral to the diencephalon (fig. 1). They include alar and basal plate derivatives defining rostral and caudal parts, whereas the aforesaid telencephalic territories consist only of alar derivatives [Puelles and Rubenstein, 2003]. The alar plate derivatives form a banded pattern of cells in the preoptic area, the supraoptoparaventricular band (SPV; containing the magnocellular neurosecretory nucleus) and the suprachiasmatic and paraventricular regions. Basal plate derivatives include the retrochiasmatic region, the tuberal (infundibular) hypothalamus (rostrally), and the mammillary region (caudally). Because of the already mentioned brain flexure, the ‘transverse’ sections through these regions are basically ‘horizontal’ sections (fig. 1). This scheme follows that proposed and analyzed for anurans [Puelles et al., 1996, Milán and Puelles, 2000] and extended for the case of urodeles [Morona and González, 2008]. Actually, in the brain of Dermophis the distribution of CB and CR helped the identification of these regions.
The localization of CBir cells included the preoptic area just beneath the anterior commissure and around the preoptic recess of the third ventricle (fig. 2g–i, 8a–d, 10a). They continued in the SPV and extended to regions adjacent to the medial amygdala (fig. 2i, j, 4i, 8d, 10d). Intense CBir neurons were observed in the suprachiasmatic region, primarily in the caudal part (fig. 2k, l, 4i, 8c, d, 10d). A distinct population of CBir cells was also located in the paraventricular nucleus of the alar part of the caudal hypothalamus (fig. 2k, l, 4i, 8a, d). Within the basal part of the hypothalamus, the topologically rostral portion contained CBir cells in the retrochiasmatic region and the tuberal nuclei, mainly in the intermediate tuberal nucleus (fig. 2m–o). In the caudal hypothalamus, the CBir cells located in the mammillary and superficial mammillary region were particularly abundant; their projections were observed to be directed toward the thalamus and the medial telencephalic wall, and they formed a cell population that continued caudally into the basal plate of the diencephalon (fig. 2n–p, 4k, 5b, e, 8a–d).
With regard to the CRir cell populations, they were specially abundant in the anterior preoptic area and the SPV, which were dorsolaterally continued with the cells of the medial amygdala (fig. 3h, 9a–c). CRir were particularly abundant in the suprachiasmatic region (fig. 3j, k) and mainly occupied a peripheral position in the periventricular cell layer (fig. 5a, c, 10d). A striking group of CRir cells was found in the paraventricular nucleus of the alar caudal hypothalamus that limited with the caudal diencephalon that was almost devoid of labeling (fig. 3j, k, 5a). Within the basal hypothalamus, CRir cells were conspicuous in the tuberal region in which the medial, intermediate, and caudal nuclei showed intensely labeled cells (fig. 3l, m, 4l, 9a–d). Caudally, CRir neurons were present in the mammillary band (fig. 9a–d), mainly forming a strip of cells in the named superficial mammillary nucleus, located laterally to the nucleus of the periventricular organ (fig. 3m). It is important to note that the optic chiasm is rudimentary in Dermophis and the optic tracts are very reduced and were not labeled for CB or CR.
Diencephalon
This portion of the gymnophionan brain, as mentioned above, is almost vertically oriented due to the sharp flexure of the brain (fig. 1). Thus, the 3 neuromeres that constitute the diencephalon (p1–p3) in conventional transverse sections appear one above the other. Sagittal sections are better suited for understanding the actual neuromeric arrangement of the diencephalic cell groups in Dermophis (fig. 8, 9). In fact, CB and CR immunohistochemistry has been especially useful for identification of the diencephalic neuromeres.
Within the rostral segment (p3), the prethalamic eminence occupies the dorsal position and it showed a CBir cell group identified as the bed nucleus of the stria medularis (BSM) by its relation with the fiber tract that crosses in the habenular commissure (fig. 2j, 4i, 8b). More ventrally, the prethalamus constitutes the alar part of p3 (previously named ventral thalamus) and in its rostral part (Vr) showed a group of CBir cells (fig. 2k–m, 4i), whereas CRir cells in Vr were located more medially and extended dorsally into regions medially situated to the BSM (fig. 3i, 4j). In clear contrast, the caudal part (Vc) was almost totally devoid of labeled cells (fig. 2k–m, 3j, k, 8c, d, 9b, d). Only at its most caudal levels was a band of CRir cells found in Vc that, by comparison with anurans and urodeles, was named an intercalated nucleus (IC in fig. 3l, 5a, 9c). Abundant CBir cells occupied the basal part of p3 in the area generally identified as the retromammillary region (fig. 2n, o, 5e, 8). Within this region, a periventricular CBir cell group was very prominent and continued dorsally just between p3 and p2 in what has been termed the zona incerta by comparison with its content in dopaminergic cells [Milán and Puelles, 2000]. The CBir cells located ventrally in the basal plate formed a continuation with the cells in the mammillary region (fig. 8d). The basal plate of p3 also contained numerous CRir cells that were primarily located in the periventricular region (fig. 3m, n, 9a–d).
The intermediate diencephalic segment p2 contains dorsally the large habenulae and the thalamus (previously named dorsal thalamus), whereas the posterior tubercle is located in its basal plate (fig. 1). The dorsal p2 showed a strikingly complementary pattern of CB and CR immunoreactivity (fig. 10e). Thus, CBir cells and fibers conspicuously occupied the dorsal habenula (fig. 2h–l, 4m), whereas CRir cells and fibers were specifically distributed in the ventral habenula (fig. 3g, h, 4n). From the dorsal habenula, which forms a large protrusion that bends caudally on top of the dorsal part of p1, CB-labeled fibers coursed in the fasciculus retroflexus toward the ventral tegmentum of the upper brainstem, with this tract serving as an indication for the boundary between p2 and p1 (fig. 5b, e, 8a, c, d). No asymmetry between right and left habenulae was noted in Dermophis on the basis of CB and CR labeling. CR immunohistochemistry was particularly suited for staining of the thalamus (fig. 3j–m, 4o, 5a, c, d, 9a, c, d, 10f), whereas only subgroups of cells contained CB (fig. 2k–n, 4i, j, 5b, 10f). In keeping with the terminology used in anurans and urodeles, the rostral thalamic region is named the anterior (A) nucleus and the large caudal region is named the central (C) nucleus. CBir cells were specifically located in A and formed a compact group rostrally (Ar) and a disperse group caudally (Ac). This caudal population overlapped with a larger population of CRir cells (fig. 10f). However, probably the most conspicuously stained brain region for CR was the central nucleus (fig. 4o, 5a, c, d. 9a–d, 10f). Between the CR-positive ventral habenula and the thalamus a nonstained territory was identified as the subhabenular region (fig. 8c, 9c). The intensely CR-labeled thalamic cells send their axons into the lateral forebrain bundle toward the subpallium, whereas axons from the thalamic CBir cells, and also CRir cells, organize a concise thalamohypothalamic tract (fig. 2l, 3j, 5a, b, 9d). In the basal plate of p2 (region of the posterior tubercle) CBir cells were more abundant than in the alar plate (fig. 2p, 5e). CRir cells were also numerous but more dispersely distributed than in the thalamus (fig. 3n, 9c, d) and occupied preferentially the periventricular zone, next to the CBir cells (compare fig. 2p, 3n).
The alar plate of the neuromere p1 (pretectum) has been reinterpreted in amphibians and 3 main parts have been distinctly identified (as in amniotes): rostral precommissural (Pc), caudal commissural (Co), and intercalate juxtacommissural (Jc) parts [Morona et al., 2011]. This tripartite scheme could be identified in Dermophis on the basis of CB and CR distribution (fig. 1). A small group of intensely CBir cells was located close to the ventricle in the dorsal portion of the Pc (fig. 2n, 8b, 10f), whereas scattered CRir cells were found in this part (fig. 3m, 4o). Due to the restricted location of the labeled cells in the Pc, most transverse sections (actually oblique through p1) did not show immunoreactive cells in this part of the pretectum (fig. 5b–d). The Jc was particularly highlighted by a CBir cell population (fig. 2m, n, 8c, d, 10f), whereas CRir cells in this pretectal subdivision were more restricted (fig. 3l, 5c, 10g). Particularly Co, which occupies the whole length of the dorsal part of p1, contained a large population of small CRir neurons that helped to the identify its boundaries (fig. 4o, 5c, d, 9b, d).
Mesencephalon
This brain region in gymnophionans represents a narrow tube whose configuration is complicated by the pronounced ventral concavity of the longitudinal brain axis. Most of its cells are arranged as a more or less compact periventricular layer, and the distribution of CB and CR has been of great help in highlighting subdivisions. Rostrally, the diencephalomesencephalic boundary passes through the posterior commissure, whereas the transverse isthmo-mesencephalic limit passes behind the trochlear nucleus.
In our analysis, we considered the mesencephalon as a single brain segment that can be further subdivided into 4 longitudinal columns (bands), as previously proposed for amniotes [Díaz et al., 2000] and adapted for anuran and urodele amphibians [Morona and González, 2009]. Thus, the model identifies: (1) the dorsal band, which contains the optic tectum (OT) and 3 ventricular subdivisions named the griseum tectale (GT), the intermediate area, and torus semicircularis (Ts) in rostrocaudal order; (2) the lateral band, which forms the ventral margin of the alar plate and contains the anterodorsal (Ad), posterodorsal (Pd), laterorostral, and laterocaudal (LC) nuclei; (3) the basal band, which constitutes the basal plate or tegmentum proper and contains the anteroventral (Av) and posteroventral (Pv) nuclei, the red nucleus (RN), and the oculomotor nucleus (III), and (4) the medial band, which contains the ventral tegmental area and represents the mesencephalic floor plate.
Rostrally in the mesencephalic dorsal band, the GT possessed numerous small round CBir cells in the ventricular and periventricular regions (fig. 2n, 8) and only some scattered CRir cells located more superficially (fig. 3m, 9a, c). It is distinct from the adjacent pretectum because of the gap of the posterior commissure and the absence of CBir cells and presence of highly packed CRir cells in the Co (fig. 2n, 3m, 8c, 9c). Caudal to the GT, the OT appeared narrow and poorly developed with restricted lamination, although it showed considerable rostrocaudal extension, as seen in saggital sections (fig. 8, 9). Rostrally it embraces the GT laterally, and caudally it extends far more than the mesencephalic tegmentum, covering the rostral rhombomeres (fig. 2o–v, 3m–s). The distinct CBir and CRir cells and fibers revealed some degree of lamination in the OT of Dermophis, but different layers were not completely segregated (fig. 5e, 6a, b). The distribution of the CBir-labeled structures extended all along the OT and formed a mixed population of weak and strong CBir cells (fig. 5e, 6a, d, 8a, b) which were more abundant and intense in the medial region of the OT. Also some CBir fibers were observed in an intermediate position within the thick fiber layer, leaving the outer and inner sublayers free of labeling (fig. 2q, arrowhead in 8a). Noticeably, CRir structures showed a more restricted distribution. Less numerous CRir tectal cells were distributed just beneath the thick fibrous layer (fig. 3o–r, 6b, 9a–d) and were more densely grouped in the caudolateral portion of the tectum (fig. 6b). These cells were weakly CRir and were located in more lateral positions than the CBir cells (fig. 6a, b). Also some polygonal cells with large dendritic processes entering into the fiber layer were observed (fig. 6b). It is important to note that the retinal fibers that occupy the superficial part of the fiber zone [Himstedt and Manteuffel, 1985] were not labeled for CB or CR.
In gymnophionans there is not a clear distinction between the tectum proper and the torus semicircularis, and different toral nuclei are not distinguished [Roth et al., 1993]. As observed in sagittal sections, the rostrocaudal enlargement of the mesencephalic dorsal band also shaped the topologically caudal torus semicircularis, appearing as a thin structure lateroventral to the OT (fig. 2p–u, 3o–r, 8b–d, 9c, d). This structure showed a characteristic differential distribution of CB and CR in a mostly complementary pattern, although some overlapping regions were also observed. CBir was more abundant in the caudal and lateral toral regions (fig. 2p–u, 6a, d, 8c), whereas CRir structures occupied more rostral regions (fig. 3o, p, 9c, d).
The lateral band of the mesencephalon showed distinct CBir and CRir cell populations that delineated 2 clearly separated rostral and caudal divisions. Both proteins were abundant in cells of the anterodorsal nucleus (fig. 2o, 3n, 8, 9) and in the caudally located posterodorsal nucleus (fig. 2p, 3o, 5e, 8a, 9c), in which CRir was more abundant, particularly in the rostral half (fig. 9c). Additionally, a large laterocaudal group of small CBir neurons was observed in a migrated position in the lateral band, just rostral to the limit with the isthmus (fig. 2q–u, 5e, 6a, d, 8d). This nucleus has been named the laterocaudal mesencephalic nucleus by comparison with its counterpart similarly identified in reptiles [Díaz et al., 2000] and other amphibians [Morona and González, 2008].
The basal band or tegmentum is much less developed than the previous alar bands because the accentuated mesencephalic flexure and the high rostrocaudal enlargement of the alar bands produce a wedge shape of the mesencephalon (fig. 1). In this division, numerous CBir and CRir cells were found in the anteroventral nucleus (fig. 2o, 5e, 8a, c, 9a–c). Noticeably, the CBir cells occupied more dorsal positions in this region and were less conspicuous than the CRir cells (fig. 8a–c, 9a–c). Numerous CBir and CRir cells were also found in the posteroventral tegmental nucleus. The CRir cells were widely distributed, whereas CBir cells were grouped slightly more dorsally and closer to the ventricle than the CRir cells, although many of them were intermingled (fig. 2q, 3o, 8a–c, 9a–c). Finally, in the medial band, some sparse CBir and more abundant CRir cells were observed in a ventral position that contains dopaminergic cells compared to the ventral tegmental area [González and Smeets, 1994] (fig. 2q, r, 3o, 10i).
Isthmus
The classically considered isthmus or isthmic region is here considered a single segment currently named rhombomere 0 (r0); in Dermophis it is curved because, besides the brain flexure, the isthmo-mesencephalic boundary is markedly oblique, as observed in sagittal sections (fig. 1, 8, 9). Based on CB and CR immunohistochemistry it was possible to propose the most likely limits of this segment and distinguish some of its cell groups. In the alar region of r0, the isthmic nucleus is poorly developed in Dermophis [González et al., 2002a] and was distinct due to the lack of CBir and CRir elements (fig. 2s–v, 3p–r, 6a, c–e, 8d, 9d). Ventral to the isthmic nucleus, in the alar plate, there was a group that we named the posterodorsal isthmic nucleus (Pdi), following the nomenclature used in anurans and urodeles [Morona and González, 2009]. This nucleus contained a few CBir cells rostrally (fig. 6a) that were more numerous, packed, and intensely stained at caudal levels (fig. 6d). Within the basal plate, a distinct CBir group of periventricular cells was stained along r0, close to the alar-basal boundary (fig. 2s, 6a, d, 8a, d). This group was named the posteroventral isthmic nucleus (as in urodeles) and highlighted the isthmo-rhombencephalic boundary because these CBir cells continued caudally and laterally through a sharp transverse line (fig. 8c, d). CRir neurons were also found in the ventral portion of the posteroventral isthmic nucleus along r0 (fig. 3p, q, 6c). The caudal boundary of the isthmic segment was also evidenced by the intense CBir in the interpeduncular neuropil located in the r1 segment (fig. 8c, d).
Hindbrain
This caudal part of the brainstem is formed by rhombomeres 1–7 (r1–r7) and a large r8, which is not clearly defined and probably represents more than one segment [Cambronero and Puelles, 2000]. We evaluated the spatial pattern of CB and CR immunoreactivity under this scheme, attending as well to the longitudinal organization within each segment; therefore, we will refer to alar and basal derivatives.
Alar Derivatives
The cerebellum originates in the rostral rhombic lip that develops bilaterally from the dorsolateral portions of the alar plates of r1 and r2 forming the cerebellar plate, and the subsequent growth causes the two rhombic lips to fuse in the midline to produce the lateral auriculae and the medial corpus cerebelli. In gymnophionans this structure has not been clearly identified cytoarchitectonically [Kühlenbeck, 1922, 1975, 1978] and only some laterally located cells in r1 were proposed in Dermophis as a cerebellar nucleus because of their connections [Sánchez-Camacho et al., 2001]. In the other amphibian orders, it was corroborated that CB is constant in Purkinje cells in all species studied, particularly in the auricular lobes [Uray et al., 1998; Uray and Gona, 1999; Morona and González, 2009]. In Dermophis, clearly intense CBir cells appeared rostrally in the alar plate of r1. This region, located laterally in the dorsal and rostral edge, appeared as the most intense labeled group for CB in r1 and might represent a cerebellar primordium in the brain of Dermophis (Cb in fig. 2v, 7a). The CB-labeled cells were arranged in a thin band that matched well with the gap left by the cholinergic and nitrergic cells of the laterodorsal tegmental nucleus (LDT) [González et al., 2002a, b] and the CRir cells of the parabrachial area (Pb) (fig. 7b). Different cell types could not be distinguished in this reduced structure.
Within the alar region in r1, CBir and CRir cells were found in the previously described LDT [González et al., 2002a, b] and in close proximity to the locus coeruleus [González and Smeets, 1994] (fig. 2t, 3r, 6e, f). All through the length of the rhombencephalon, the alar plate contains the cell masses related to the octavolateral system in gymnophionans [Fritzsch, 1988; Fritzsch and Wake, 1988]. The organization of this area in adult Dermophis has not been studied but, by comparison with members of the same family, Caeciliidae (Boulengerula boulengeruli), in the continuous mass of undifferentiated periventricular cells the octaval projections would occupy the dorsalmost aspect of the alar plate because the lateral line system is not present [Fritzsch and Wake, 1988]. Only sparse CBir and CRir cells were detected along the alar plate of rhombomeres r1 and r2 and, in general, the rostral portion of the alar plate showed CRir neurons (fig. 3p–s, 6f) that were more numerous than the CBir cells (fig. 2s–v). Noticeable, intense CBir fibers were found in the alar r1 and extending into r2, probably next to the principal sensory trigeminal nucleus (fig. 8a). In the caudal rhombencephalon, labeled cells related to the octaval afferents were scarce and CBir fibers were labeled in the lateral aspect of the rhombencephalon that, along their course, were related to CBir cells located more medially and could be part of the descending trigeminal nucleus (fig. 2u–x). At caudal segments, a conspicuous cell population was labeled for CB and CR in the alar rhombencephalon that corresponded to the nucleus of the solitary tract (fig. 2w, x, 3u, v, 7h–k). Also in the caudal alar region intense CBir cells were identified as the dorsal column nucleus by comparison with similarly located and stained cells in anurans and urodeles (fig. 2y, 7i, k, 8).
Basal Derivatives
In the basal rhombencephalon, distinct CBir and CRir populations were revealed. In the medial part of the rostral r1, the interpeduncular nucleus contained CBir cells that were located above the CBir interpeduncular neuropil (fig. 2s, t, 6d, e, 8a, c). A distinct group of CBir cells occupied the medial dorsal portion in the griseum centrale (Gc), highlighting the rhombomeric boundaries, and were especially useful for identification of the rostral and caudal limits of r2 (fig. 2u, 6a, e, 8b). These CBir small cells of the Gc were peripherally located with cell processes medially oriented to the central neuropil (fig. 2u). Within the same medial position the disposition of the CRir cells in the Gc was similar, but actual colocalization was not demonstrated (fig. 3q, r, 9b). Migrated cells in the basal region in r4 and r5 were labeled for CB and, to a lesser extent, for CR (fig. 2v, 3s, 7e, f).
Along the rhombencephalic reticular formation, CBir and CRir cells were labeled. In general, CBir and CRir cells were found in several groups in the nucleus reticularis superior (fig. 2u, 3r, 6e, 7c), in the nucleus reticularis medius (fig. 2v, w, 3s, t, 7c–f), and in the nucleus reticularis inferior (fig. 2x, 3u, v, 7g, h) that, as seen in sagittal sections, were arranged in regular clusters that respected rhombomeric boundaries (fig. 8c, d, 9a–d).
Low numbers of large cells were CBir and CRir within the basal plate that, compared with the distribution of ChAT immunoreactivity [González et al., 2002a], seemed to correspond to trigeminal and facial motor neurons (fig. 2u, w, 3r, 7d, e). This fact was supported by the observation of axons in the facial motor root (arrowhead in fig. 7e).
Spinal Cord
CB and CR labeled segregated populations in the rostral segments of the spinal cord that were analyzed. Both proteins labeled cell subpopulations of the dorsal and ventral gray horns (or fields since clear horns are not evident) and fibers in the different funiculi. CB was distributed in small rounded cells in the dorsal horn that possessed thin processes which were dorsally oriented (fig. 2z, 7l). The ventrally located CBir cells were evenly distributed. CRir spinal cells were located slightly laterally within the dorsal gray (fig. 3w) and the population in the ventral gray consisted of some polygonal cells. CBir and CRir fibers were primarily labeled in the dorsolateral funiculus and, to a lesser extent, CBir fibers were also observed in the lateral funiculus (fig. 2z, 7l).
Double Immunohistochemistry
The distribution of CBir and CRir cells in the brain of Dermophis suggested the possibility of colocalization in many regions, as observed in anurans and urodeles [Morona and González, 2008, 2009]. This prompted us to conduct double immunohistochemistry for the simultaneous detection of both proteins in the same sections (fig. 10). However, although CBir and CRir cells are intermingled in many regions, actual colocalization of both proteins in the same neurons could be demonstrated only in a few sets of neurons. Thus, double-labeled (CBir/CRir) neurons were detected in the preoptic area (fig. 10a–c) within the region that is close to the telencephalon and far from the alar-basal boundary. The caudal suprachiasmatic region also contained double-labeled cells close to the limit with the rostral subnucleus (fig. 10d).
The double immunofluorescence not only revealed double-labeled cells but also served to highlight the boundaries and subdivisions of some regions, i.e. specially outstanding within the diencephalic prosomeres. CB and CR showed a complementary pattern in the dorsal and ventral subdivisions of the habenula (fig. 10e) as well as in the thalamus (p2), where CB marked the rostral portion (Ar and Ac) that contained fewer CRir cells. Some of these neurons were double labeled just in the anterior caudal subdivision (fig. 10f).
CB also marked the juxtacommissural subdivision in the pretectum where some of the weak CRir cells were double labeled (fig. 10g). Although extensive overlapping was observed in some regions, as in the isthmic tegmentum and in the Gc (fig. 10h, h’, i), most cells were single labeled (fig. 10h, h’) and just a few cells contained both proteins in the posteroventral isthmic nucleus (fig. 10i). Other subpopulations of double-labeled cells in the rhombencephalon were more numerous. Rostrally, in the alar plate numerous rounded CBir/CRir cells were revealed in the octaval column (fig. 10j) and caudally in the region of the nucleus of the solitary tract (fig. 10k).
Discussion
In the present study we provide a detailed and comprehensive analysis of the distribution patterns of CB and CR in the central nervous system of the gymnophionan amphibian D. mexicanus. This work complements the previous studies performed in anurans and urodeles where a similar approach was used [Morona and González, 2008, 2009]. These patterns observed for representative species of the three amphibian orders share characteristic features with a notable order specificity but also show particularities in each species. Besides these differences, CB and CR immunoreactivities proved to be suitable markers for delimiting particular brain areas from their neighboring territories, which are otherwise indistinguishable, and allowed the identification of labeled cell groups within certain regions that support distinct subdivisions.
For understanding the distribution patterns for CB and CR in Dermophis, we used the previous studies of Milán and Puelles [2000] and Morona and González [2008, 2009] as a guide for the interpretation of the novel information in this most neglected amphibian order.
In the following sections, the general organization and variations of the CBir and CRir structures in amphibians are discussed, primarily pointing out the distinct features found in Dermophis. We specifically attend to the current ideas for the regionalization of the forebrain and brainstem. Although it is not the aim of the present study to deal extensively with the detailed distribution of these proteins in other vertebrates, some comments will be made when needed to assess common versus specific features of amphibians and, in particular, of Dermophis.
Olfactory Bulbs
A feature shared by the three orders of amphibians is the abundant CR immunoreactivity found in primary olfactory and vomeronasal fibers and in many cells and fibers distributed equally in the MOB and AOB, with an abundance of labeled neurons in the internal granular cell layer [Marín et al., 1998; Necchi et al., 1999; Brox et al., 2003, Mühlenbrock-Lenter et al., 2005; Morona and González, 2008]. However, a difference with anurans and urodeles was noted in Dermophis where periglomerular and mitral cells were not stained for CB or CR [Morona and González, 2008].
The presence of CR in the olfactory receptors, olfactory nerve fibers, and bulbar glomeruli seems to be a characteristic shared by anamniotes [Kerschbaum and Hermann, 1992; Díaz-Regueira and Anadón, 2000; Pombal et al., 2002; Castro et al., 2003, 2006; Huesa et al., 2006; present results]. As in amphibians, diversity exists with regard to the presence of CR in bulbar cells of fish because both interneurons and mitral cells contain CR in lampreys [Pombal et al., 2002] and some teleosts [Porteros et al., 1997; Díaz-Regueira and Anadón, 2000], whereas only scarce CRir interneurons were demonstrated in a chondostrean fish [Huesa et al., 2006] and no CRir cells were detected in the zebrafish and catfish olfactory bulbs [Castro et al., 2006; Jadhao and Malz, 2007]. The numerous studies conducted in different mammalian species also show enormous diversity in the content of CB and CR in bulbar cell populations [Alonso et al., 1993, 1995; Malz et al., 2000; Jia and Halpern, 2004; Parrish-Aungst et al., 2007]. However, it is a shared feature that the periglomerular cells are always rich in CB and CR [Briñón et al., 1992; Alonso et al., 1993; Toida et al., 1998; Kakuta et al., 2001; Jia and Halpern, 2004; Kosaka and Kosaka, 2007], whereas species differences exist regarding the localization of these proteins in granule cells [Kakuta et al., 2001; Jia and Halpern, 2004].
Pallium
In concordance with the observation in anurans and urodeles, the pallium of Dermophis possesses low numbers of CBir cells and, in contrast to the members of the other two orders, almost completely lacks CBir in the large medial pallium [Morona and González, 2008]. In turn, the population of CRir cells located in the dorsal and lateral pallial areas of Dermophis largely resembles the situation found in the two urodele species studied, highlighting the closer resemblance between the pallial regions of urodeles and gymnophionans, as previously proposed [Northcutt and Kicliter, 1980]. Moreover, the pattern of fiber labeling in the pallium is also similar in urodeles and Dermophis, in which a long fiber bundle and terminal field intensely stained for CR occupies the superficial zone of the medial, dorsal, and, to a lesser extent, lateral pallial regions [Morona and González, 2008]. It is noteworthy that the pattern of distribution of CR in Dermophis helped to highlight the boundary between the medial and dorsal pallia, which was controversial in classical studies [Kühlenbeck, 1922; Welsch and Tan, 1979; Northcutt and Kicliter, 1980].
Cortical subpopulations of neurons in amniotes are distinctly labeled for CB and CR, making the study of the distribution of these CBPs of special significance for establishing cell types and cortical subdivisions [Dávila et al., 1997, 1999; DeFelipe, 1997; Chiry et al., 2003; Krutzfeldt and Wild, 2004; Suárez et al., 2006]. In general, different types of nonpiramidal neurons are stained for each protein and display different neurochemical characteristics [Martínez-Guijarro and Freund, 1992; DeFelipe, 1997; Guirado and Dávila, 1999; Wild et al., 2005; Suárez et al., 2006; Rahman and Baizer, 2007]. Strikingly different to amniotes are the results described for nonamphibian anamniotes in which CBP-containing cells are absent or very scarce in pallial regions [Díaz-Regueira and Anadón, 2000]. However, as we have observed in Dermophis, CR is significatively present in pallial subdivisions of the zebrafish, and the localization of CRir has shown that cytoarchitectonic subdivisions of the dorsal telencephalon can be differentiated by their content in CRir structures [Castro et al., 2006].
With regard to the innervation of the pallium by CRir fibers in the three amphibian orders, it should be mentioned that it largely resembles the innervation of cortical regions of reptiles [Dávila et al., 1997, 1999]. However, in reptiles the CRir fibers and terminals that formed the characteristic cortical band were suggested to have originated in the cholinergic basal telencephalic cell population because terminals containing acetylcholine distribute in the reptilian cortex forming a band similar to that observed for CR (and CB) [Dávila et al., 1997]. In Dermophis, as in other amphibians, although a basal forebrain cholinergic cell group has been demonstrated, the pattern of innervation of the pallium does not resemble that observed for CR or CB [Marín et al., 1997; González and López, 2002; González et al., 2002a; Sánchez-Camacho et al., 2006]. According to our results in Dermophis, this pallial innervation most likely arises in intrapallial neurons or in the abundant CRir thalamic cells, as also proposed for anurans and urodeles [Morona and González, 2008].
Septum
The large septal region in Dermophis formed, together with the medial pallium, a bulge into the lateral ventricle that was virtually devoid of CR- and CB-containing cells and fibers. Only the rostralmost part of the septum contains a distinct CBir cell population that by its position and in comparison with results in anurans [Morona and González, 2008] may represent the counterpart of the anuran postolfactory eminence, as proposed earlier [Northcutt and Kicliter, 1980]. The actual boundary between the medial pallium and the septal region located just below is hard to distinguish and becomes evident only when immunohistochemistry for the detection of several peptides is used [González et al., 2002b; López et al., 2006, 2007]. The largest septal region of gymnophionans was considered homologous to the lateral septal nucleus of anurans on the basis of its abundant catecholaminergic innervation and lack of cholinergic and GnRH immunoreactive cells [González and Smeets, 1994; Pinelli et al., 1997; Rastogi et al., 1998; Ebersole and Boyd, 2000; González et al., 2002a]. This region almost totally lacks CBir or CRir cells or fibers, as was also observed in the lateral septal region of urodeles [Morona and González, 2008]. Differently, abundant septal CRir and CBir cell populations in anurans distribute differently within distinct nuclei [Sánchez-Camacho et al., 2003, 2006; Endepols et al., 2006]. Interestingly, the caudally located and shrunken septal region of Dermophis would correspond with the medial septal nucleus which contains, in addition to the CRir cell population demonstrated in the present study, GnRH immunoreactive cells and cholinergic and nitrergic neurons, as in anurans and urodeles [Marín et al., 1997; Rastogi et al., 1998; González et al., 2002a, b]. It should be kept in mind that in urodeles, while Pleurodeles is virtually devoid of labeled cells in the septum, Ambystoma possesses both CRir and CBir cells restricted to the medial septal region [Morona and González, 2008]. Therefore, as in all other brain regions, the situation found in Dermophis should not be generalized for all other gymnophionan species. Major differences are noted in the lateral septal area in terms of CB and CR immunoreactivity between amniotes and amphibians [Riedel et al., 2002], whereas the presence of CRir cells in the medial septum is a shared feature of tetrapods [Kiss et al., 1997; Borhegyi and Freund, 1998]. Moreover, considering the scarce data available for other anamniotes, the CRir cells localized in the ventrolateral telencephalic region of the trout, together with other neurochemical data, support that this nucleus of teleosts is a homolog of some medial septal neuronal populations in tetrapods [Castro et al., 2003].
Basal Ganglia
In the basal ganglia of gymnophionans only the striatum proper could be distinguished in studies based on classical methods [Kühlenbeck, 1922; Northcutt and Kicliter, 1980]. By means of immunohistochemistry to unravel the distribution of catecholamines, acetylcholine, and several peptides, a nucleus accumbens was proposed rostrally in the hemispheres, just medial to the ventral tip of the lateral ventricles [González and Smeets, 1994; González et al., 1994; González et al., 2002a, b; López et al., 2006, 2007]. However, tract-tracing studies and specific stainings to unravel possible pallidal zones in the basal telencephalon have not been conducted in gymnophionans. Our study has corroborated that the striatum of Dermophis is a large structure in the ventrolateral hemisphere that contains moderate numbers of CBir and CRir cells and is conspicuously innervated by terminals labeled mainly for CR. This distribution basically coincides with those observed in anurans and urodeles [Marín et al., 1998; Brox et al., 2003; Morona and González, 2008]. However, the amount and intensity of CR staining in the striatal neuropil is less conspicuous in Dermophis, suggesting that the thalamostriatal CR-containing projection is not as abundant as in the other amphibians. The distribution of CBir and CRir cells in the striatum and in the region identified as possible nucleus accumbens supports the idea of an especially well-developed striatal region in gymnophionans, as previously evidenced by the distribution, for example, of catecholaminergic and nitrergic structures [González and Smeets, 1994; González et al., 1994; 2002a].
As previously noted, information about the localization of pallidal structures in the gymnophionan basal ganglia is lacking. In contrast, on the basis of the localization of the transcription factor Nkx2.1 in anurans and urodeles, the position of the pallidal region has been identified [González et al., 2002c, d; Moreno and González, 2007a] as in amniotes [Price, 1993; Sussel et al., 1999; Puelles et al., 2000]. By comparison, the putative pallidal region of Dermophis would be situated in the caudal hemisphere, close to the amygdaloid territories, and would derive from the ventromedial ventricular zone. It is noteworthy that the recently identified pallidal region of urodeles contains a particular CBir cell population [Moreno and González, 2007a; Morona and González, 2008] and similarly labeled cells are located in comparable regions of Dermophis, although their pallidal nature awaits neurochemical and hodological confirmation.
In amniotes, the distribution of the large populations of CB- and CR-containing cells has served to evaluate distinct chemoarchitectonic and functional domains within the complex organization of the basal ganglia [Holt et al., 1997; Hontanilla et al., 1998; Morel et al., 2002]. It was generally corroborated that most CRir cells in the striatum are interneurons [Bennett and Bolam, 1993; Figueredo-Cardenas et al., 1996; Cicchetti et al., 1998, 2000], whereas subpopulations of CBir-projecting cells were reported in primates [Parent et al., 1996; Cicchetti et al., 2000; Cooper and Stanford, 2002]. The characterization of the CBir and CRir striatal cells as interneurons or projecting neurons has not been investigated in any group of amphibians. Information about CBP distribution in other groups of anamniotes is very scarce, but the presence of CR has served to clarify the putative localization of the striatum in the ventral telencephalon of the trout [Castro et al., 2003]. Moreover, in a recent study of the telencephalon of lungfish, a small CBir cell population was found in the striatum that contrasted with the abundance of CRir fibers and terminals located in the striatal neuropil, thus resembling the situation found in the three amphibian orders [González and Northcutt, 2009; unpubl. results].
Amygdaloid Complex
In the present study we have tentatively differentiated 3 (lateral, central, and medial) amygdaloid regions within the caudal telencephalon in a position that almost totally corresponds with the previously named ‘pars lateralis of the amygdala’ [Northcutt and Kicliter, 1980]. This subdivision was prompted by the distinct distribution of diverse markers in the telencephalon of gymnophionans [González and Smeets, 1994; González et al., 2002a, b; López et al., 2006, 2007] and by comparison with anurans and urodeles [Moreno and González, 2006, 2007a]. The lateral amygdala was defined as a multimodal area in the ventral pallium [Moreno and González, 2004] and the actual ventral pallial origin of this region was confirmed by the expression of territorial genetic markers [Smith-Fernandez et al., 1998; Brox et al., 2004; Moreno et al., 2004]. The distinct pattern of CB and CR distribution in combination with the detection of nitrergic structures has served to identify the lateral amygdala from its neighboring pallial and subpallial regions in anuran and urodele amphibians [Moreno and González, 2004, 2007a; Morona and González, 2008]. In Dermophis, a distinct region between the lateral pallium and the striatum contains densely labeled cells and fibers for nitric oxide synthase immunohistochemistry that would correspond to the ventropallial region containing the lateral amygdala [González et al., 2002b]. In our present study, both CBir and CRir cells were clearly observed in this region, between the lateral pallium and the striatum, supporting the comparison with other amphibians. The presence of CBir and CRir cells in this region is shared by the three amphibian orders, but the pattern observed in Dermophis is more similar to that in urodeles, where the CRir cells also extended more caudally accompanied by the nitrergic neuropil, whereas CBir cells occupied only the rostral part. This situation could suggest a subdivisionwithin this area.
The central amygdala of anuran and urodele amphibians is currently interpreted, like in amniotes, as the striatal component of the amygdaloid complex [Moreno and González, 2005, 2006] and the presence of CBir and CRir cells in this region follows the pattern observed in the striatum [Morona and González, 2008]. In Dermophis, the proposed central amygdala would represent the caudal part of the large striatal region described on the basis of cytoarchitectonics [Northcutt and Kicliter, 1980] and contains CBir and CRir cells distributed as a caudal continuation of the striatal cells. Although the connectivity of this region has not been investigated in gymnophionans, in the other amphibians is particularly connected with brainstem centers [Moreno and González, 2005]. The caudal ‘striatal’ cells that were revealed to posses long descending projections in gymnophionans likely correspond to actual central amygdaloid cells [Wicht and Himstedt, 1988; Sánchez-Camacho et al., 2001].
The medial amygdala has been redefined in amphibians as the main vomeronasal center receiving secondary information from the AOB and comprises a large part of the early named pars lateralis of the amygdala in all amphibians [Northcutt and Kicliter, 1980; ten Donkelaar, 1998a, b; Moreno and González, 2003, 2006]. The pattern of CBir and CRir cells observed in Dermophis in a similar location suggests that the medial amygdala is a large structure that extends caudally in the hemisphere. The possible enlargement of this vomeronasal amygdaloid territory is not surprising considering the large AOB of gymnophionans that have been related to the presence of a specific tentacle associated with the vomeronasal organ and the vomeronasal chemoreception [Badenhorst, 1978; Billo and Wake, 1987]. This unique organ appears to be very important in the ecology of the gymnophionans, and thus primary and secondary centers of the sensory vomeronasal information are consequently well developed.
Finally, most of the previously described ‘pars medialis of the amygdala’ in the caudal telencephalon of gymnophionans has been considered here as a putative BST by comparison with the similarly located nucleus in anurans identified by its connections and neurochemistry, including the distribution of CB and CR [Marín et al., 1998; Moreno et al., 2011], which concurs with the observations made in the present study in Dermophis.
The distinct distribution of CBPs in the amygdaloid complex of diverse amniotes has served as an anatomical marker to define subterritories [Sorvari et al., 1996a, b; Kemppainen and Pitkanen, 2000; Ashwell et al., 2005; Legaz et al., 2005]. However, many species differences were described that made impossible the use of CBPs to propose homologies, although their distribution constitutes a good anatomical tool. Among anamniotes, the 3 amygdaloid regions described for amphibians have been recently proposed in lungfish and the coelacanth Latimeria columnae [Northcutt, 2008; González and Northcutt, 2009; Northcutt and González, 2011] and the distribution of CB and CR, at least in lungfish, supports their distinction. In contrast, the telencephalon in actinopterygian fish undergoes an eversion process instead of the evagination that occurs in other vertebrates; thus, the teleostean mediodorsal pallium would correspond, as a whole, to the lateroventral pallium of ‘evaginated brains’. In different works, homologies for the teleostean pallium have been postulated and recently reviewed by Northcutt [2008]. However, in amphibians and teleosts, the distribution of CBPs in the telencephalon is strikingly different and it is not possible to establish regional comparisons on the basis of distinct locations for CB and CR.
Nontelencephalic Secondary Prosencephalon and Hypothalamus
Most studies in gymnophionans have highlighted the great expansion of the hypothalamus, including the suprachiasmatic and preoptic regions, that in some cases was observed to be more distinctly organized into segregated cell groups than in urodeles [González and Smeets, 1997; Pinelli et al., 1997; Hilscher-Conklin et al., 1998; Ebersole and Boyd, 2000; Ebersole et al., 2001; Muñoz et al., 2001; Rastogi et al., 2001; López et al., 2006]. The present study has revealed that the distribution of CB and CR within this set of regions allows the distinction of most of the cell groups identified in anurans and urodeles [Milán and Puelles, 2000; Morona and González, 2008]. Thus, in the preoptic area, the basically segregated distribution of CBir and CRir cells suggests further subdivisions of this large region, as previously proposed in anurans and in concordance with results in amniotes [Celio, 1990; Fortin and Parent, 1997; Cheng et al., 2003; Huesa et al., 2006; Morona and González, 2008; Real et al., 2008]. Moreover, the subdivision of the preoptic area in Dermophis is suggested by the distinct distribution of other neurochemicals, such as tyrosine hydroxylase and nitric oxide synthase, that specifically label regions within the preoptic area distinct from those labeled for the CBPs [González and Smeets, 1994; González et al., 2002b].
Particularly abundant are the CBir and CRir cells located in the suprachiasmatic region. As already noted, the identification of a clear optic chiasm in Dermophis is arduous because the reduced number of fibers that constitute the optic nerves and only a small ridge, at best, can be localized in sagittal sections. However, the distribution of many neuropeptides and other chemical markers allows recognition of a similar suprachiasmatic ‘nucleus’ in gymnophionans that topologically corresponds to its counterparts in other vertebrates. The content of CBPs, particularly CB, in the suprachiasmatic nucleus has been related to the control of circadian rhythms [LeSauter et al., 1999; Arvanitogiannis et al., 2000; Bryant et al., 2000; LeSauter et al., 2009; Stadler et al., 2010]. This nucleus is the primary circadian pacemaker that reinforces their oscillations and synchronizes its components in response to light input [Welsh et al., 2010]. In spite of their fossorial life, light detection may still play an important role in the lives of these ‘blind’ animals for circadian entrainment or setting seasonal rhythms. Results obtained in some studies in other fossorial vertebrates support this hypothesis [Cooper et al., 1993; Herbin et al., 1994; Crish et al., 2006].
The distribution of CB and CR led us to consider the recently coined term supraoptoparaventricular region as a band between the preoptic area and the suprachiasmatic region that contains the magnocellular neurosecretory nucleus and stains distinctly for other markers [González et al., 1994; González and Smeets, 1997; Hilscher-Conklin et al., 1998; Muñoz et al., 2001; López et al., 2007]. This band is histogenetically distinctly specified during development in a manner that has been shown to be constant at least for tetrapods [Bardet et al., 2008].
The large basal hypothalamus of Dermophis shares with that of anurans and, to a lesser extent, with that of urodeles the presence of abundant CBir and CRir cells distributed in the median, intermediate, and caudal tuberal nuclei [Morona and González, 2008]. In addition, the CBir cell population located in the caudal basal hypothalamus in the lateral and mammillary region is outstanding in Dermophis, and fibers from this labeled cell group seem to originate projections to the thalamus and the medial telencephalic wall.
Comparatively, in the same manner as that described for mammals [Puelles and Rubenstein, 2003], the pattern of bands distinguished in the alar part of the hypothalamus by means of CB and CR immunohistochemistry would correspond to actual longitudinal bands because of the distinct brain flexures. Similar analysis in other groups of vertebrates would serve to highlight the constancy of these regions.
Particularly abundant CBir and CRir cells have been localized in the infundibular nuclei of mammalian and nonmammalian species [Celio, 1990; Résibois and Rogers, 1992; Rogers and Résibois, 1992; Fortin and Parent, 1997; Castro et al., 2006], which is in concordance with the results in amphibians. It is noteworthy that the mammillary projection to the anterior thalamic region in mammals is essential for spatial memory [Vann and Aggleton, 2003] and learning [Méndez-López et al., 2009]. The observed CBir fibers connecting the mammillary region with the thalamus in Dermophis might also play a role in spatial memory functions. Interestingly, some parts of the lateral mammillary nucleus has also been involved in the regulation of neurons in the respiratory center of rats’ medulla during hypoxia, and CB-containing cells could be implicated in this function [Arutiunian et al., 2009]. One of the adaptations to fossorial life is a very extended cutaneus respiration due to the extreme hypoxia and hypercapnia in some subterranean environments.
Diencephalon
In anurans and urodeles, diencephalic regions have been analyzed in relation to their content of CB and CR, and a similar regionalization was proposed in both orders [Milán and Puelles, 2000; Morona and González, 2008]. Thus the CB and CR patterns helped to define subregions in the relatively simple diencephalon of urodeles in spite of the limited neuronal migration that makes it difficult to demarcate separate nuclei. In the diencephalon of gymnophionans, the neuronal arrangement is similar to that in urodeles; thus, its study presents analogous difficulties [Schmidt and Wake, 1991; Roth et al., 1993]. Our material clearly delineates previously unidentified nuclei by the distinct distribution of CB and CR compared with previously reported immunohistochemical maps [Clairambault et al., 1994; González et al., 1994; González et al., 2002b]; however, the patterns of CB and CR distribution often vary with respect to those found in other amphibians.
Most neuroanatomical studies of the brain of gymnophionans used a classical nomenclature, indirectly assuming a straight rostrocaudal axis and 4 basic longitudinal zones: the epithalamus, the dorsal thalamus, the ventral thalamus, and the hypothalamus [Clairambault et al., 1994; Ebersole, et al., 2001;López et al., 2006, 2007]. On the other hand, the segmental interpretation was used to frame the results of very few works [González et al., 2002a, b]. However, the most recent studies of the brain of amphibians take great advantage of the use of the prosomeric model [Puelles and Rubenstein, 2003] that facilitates the interpretation of the diencephalic cell groups [Puelles et al., 1996; Milán and Puelles, 2000; Brox et al., 2004; Moreno et al., 2004; Morona and González, 2008, 2009]. Therefore, in our analysis of the distribution of CB and CR we tested the capacity of the prosomeric model to interpret the organization of the diencephalon of Dermophis. The new interpretation of the diencephalic cell groups and their localization within particular prosomeres is proposed mainly on the basis of the data about the distribution of CB and CR and their comparison with similar data in anurans and urodeles [Morona and González, 2008].
In the interpretation of the diencephalic subdivisions, the prethalamic eminence and the BSM represent the dorsal portion of the prosomere p3. The BSM of Dermophis is specifically identified by the CB immunohistochemistry, in line with the situation found in anurans and urodeles. Also shared by the three amphibian orders is the relatively scarce distribution of CBir and CRir elements in the alar part of p3 (former ventral thalamus). A striking pattern of CB and CR distribution is present in the p2 of Dermphis. CBir cells and fibers are restricted to the dorsal habenula, whereas CRir elements are located in the ventral habenula, and no asymmetry exists between right and left habenulae. Comparatively, this pattern is almost similar to that found in urodeles, although clear asymmetry was noted in the two species studied [Morona and González, 2008]. Differently, in anurans the dorsal habenula does not posses CBir elements and no asymmetry was noted [Milán and Puelles, 2000; Morona and González, 2008]. However, the three amphibian orders share the intense CR immunoreactivity present in the alar p2 (thalamus) and the codistribution with CB delineates similar subdivisions. Also in the large alar p1 (pretectum), the distinct distribution of CB and CR in Dermophis can be framed within the newly described tripartite subdivision established for anurans [Morona et al., 2011].
The labeling obtained in the diencephalon of Dermophis has served to assess a rather conservative pattern among amphibians. Comparisons with previous data in anamniote and amniote representatives highlight, again, species variations regarding the specific distribution for each CBP. However the different immunostaining allows (in most cases) clear distinction between prethalamus (p3), thalamus (p2), and pretectum (p1), as well as subdivisions within them, that respect and are well explained by the prosomeric model [Pombal and Puelles, 1999; Dávila et al., 2000; Milán and Puelles, 2000; González et al., 2002; Castro et al., 2006; Morona and González, 2008]. A main trait of the distribution of these proteins in the diencephalon is the abundant presence of CR in thalamic neurons in anamniotes [present results; Pombal and Puelles, 1999; Díaz-Regueira and Anadón, 2000; Milán and Puelles, 2000; Castro et al., 2006], whereas in amniotes CB prevails in the thalamus [Braun, 1990; Pritz and Stritzel, 1991; Pritz and Siadati, 1999; Dávila et al., 2000; Münkle et al., 2000; González et al., 2002]. Within the alar part of p1, the distribution of CB and CR in Dermophis seemed to match well with the 3 molecularly distinct anteroposterior domains already recognized in several vertebrate groups [Pombal and Puelles, 1999; Dávila et al., 2000; Milán and Puelles, 2000; Ferrán et al., 2007; Morona et al., 2011]. The large size and distinct organization of the pretectal region of Dermophis was particularly striking because this region was associated with optokinetic reflexes in amphibians [Cochran et al., 1984; Manteuffel et al., 1986; Sperl and Manteuffel, 1987; Jardon and Bonaventure, 1992, 1997] that in Dermophis are supposed to be reduced due to the minimized visual system [Himstedt and Manteuffel, 1985; Milner, 2001]. However, the pretectum is also related to orientation in prey-catching behavior [Grobstein and Comer, 1983; Ewert, 2001], which can be of special significance in Dermophis.
Mesencephalon
The identification of the rostral and caudal boundaries of the mesencephalon in Dermophis allowed us to correctly interpret different subdivisions. The diencephalomesencephalic boundary was highlighted by the CB immunoreactivity in the mesencephalic nuclei limiting with the diencephalon. This boundary is bent rostrally in the roof plate and protrudes in the basal plate of p1 at the level of the basal band.
The GT is distinct due to the particular localization of CBir and CRir cells. This region can thus be recognized in Dermophis like in other vertebrates [Puelles et al., 1996; Díaz et al., 2000; Díaz-Regueira and Anadón, 2000; Milán and Puelles, 2000; García-Calero et al., 2002]. In the OT, CBir cells were more abundant than CRir cells in all amphibians studied. In Dermophis, the CBir and CRir cells are segregated within the apparently undifferentiated periventricular cell layer. Studies on the mesencephalic tectum of gymnophionans [Schmidt and Wake, 1991] suggested that the tectum of these animals possesses an ‘intermediate’ degree of morphological complexity between anurans and urodeles, and the distribution of the studied CBPs support this idea because practically no segregation was found in urodeles and a complex distribution of CBir and CRir cells was observed in anurans [Morona and González, 2009]. If the situation in Dermophis is similar to that in Ichthyophis[Himstedt and Manteuffel, 1985] the retinal axons in the tectum are restricted to the medial and superficial zone and, therefore, they are not labeled for CB and CR. This is in striking contrast with the strong CR labeling in the retinorecipient tectal layer in anurans and urodeles [Necchi et al., 1999; Morona and González, 2009]. Moreover, the CR content of retinofugal fibers seems to be a common characteristic in other vertebrates [Rogers and Résibois, 1992; Leuba and Saini, 1997; De Castro et al., 1998; Díaz-Regueira and Anadón, 2000; Soares et al., 2001; Kang et al., 2002; García-Crespo and Vecino, 2004; Puelles et al., 2004; Castro et al., 2006; Lee et al., 2006; Jadaho and Malz, 2007; Morona and González, 2009]. The CRir fibers in the tectal retinorecipient layer have been correlated with the presence of abundant CRir ganglion cells in the retina [Gábriel et al., 1998; Morona et al., 2007b], but the lack (or presence) of CRir ganglion cells in Dermophis has not been investigated. Finally, in the OT of Dermophis trigeminal mesencephalic cells were not labeled for CB or CR as in urodeles and some anurans; actually, only in Xenopus isa subpopulation of trigeminal mesencephalic cells CBir, and most of these neurons project to the spinal cord [Morona et al., 2006a].
In gymnophionans the torus semicircularis is not conspicuous and its distinction from the OT is not clearly marked cytoarchitectonically [Roth et al., 1993]. However, the analysis that we have conducted based on the longitudinal organization of the mesencephalon that is better interpreted in sagittal sections has allowed its distinction in Dermophis, mainly due to the pattern of CB and CR distribution. Thus, in the topologically located dorsal band, the torus semicircularis possesses distinct CRir cells rostrally and CBir cells caudally and this segregated pattern largely resembles that observed in anurans where the torus is highly developed [Morona and González, 2009]. In anurans, the sensory significance of the nuclei that comprise the torus semicircularis is known and the distribution of these proteins in toral nuclei could be related to the processing of acoustic or lateral line information. However, adult Dermophis have most likely completely lost the lateral line system [Fritzsch, 1988] and, therefore, the torus semicircularis would be a main octaval sencondary center but acoustic and vestibular regions have not been determined. At this point we can assume toral parcelation as observed for CB and CR distribution, but the implication of each subzone in a specific type of sensory information has to be investigated. Comparatively, the distinct distribution of CB and CR has been useful for analysis of particular cell groups in the torus not only in amphibians but also in the chick [Puelles et al., 1994].
Due to the cephalic flexure, the tegmental bands of gymnophionans are reduced and show a much shorter rostrocaudal extent than the dorsal bands. Distinct CBir and CRir cell populations show that the tegmentum can be easily subdivided into anterior and posterior nuclei, in line with the organization found in anurans and urodeles [Morona and González, 2009]. Also abundant tegmental cell populations containing CB and CR have been described in different vertebrates [García-Segura et al., 1984; Celio, 1990; Résibois and Rogers, 1992; Díaz-Regueira and Anadón, 2000; Castro et al., 2006]. In particular, 3 main tegmental groups have been especially addressed in several studies: the RN, the oculomotor nucleus, and the catecholaminergic A9–A10 group (substantia nigra and ventral tegmental area) [Brauth et al., 1988; Celio, 1990; McRitchie et al., 1996; Wang et al., 1996; De la Cruz et al., 1998; Verney et al., 2001]. The RN is a major component of the rostral portion of the basal mesencephalic band [Díaz et al., 2000] and it was tentatively identified in Dermophis by its contralateral projection to the spinal cord [Sánchez-Camacho et al., 2001], but these cells were clearly migrated from the ventricle into a position devoid of CBir and CRir cells [present results]. The oculomotor nucleus of Dermophis is formed by a low number of motoneurons arranged close to the ventricle in the basal band [González et al., 2002a] and, according to the present results, only partial colocalization with the CRir cells (the most ventrally located in the ventral band) could exist, although corroboration is needed by double-labeling techniques. In other amphibians variability has been found because CB is present in some oculomotor cells of anurans, whereas CR and CB are located in subpopulations of oculomotor neurons in urodeles [Morona and González, 2009]. Taking into consideration that double labeling was not performed in other studies, some oculomotor neurons in mammals were described to contain CB in rats [Celio, 1990] and cats [De la Cruz et al., 1998], and no CRir oculomotor cells were found [Arai et al., 1991]. In contrast, CB is absent in oculomotor cells in birds [Fujii and Lucaj, 1993], and CR is present in this nucleus in teleosts [Díaz-Regueira and Anadón, 2000; Castro et al., 2006]. Therefore, the variability shown for amphibians is also common among other vertebrates.
Finally, in the mesencephalon of gymnophionans, a strikingly large group of catecholaminergic cells (putative dopaminergic) was described in the medial band [González and Smeets, 1994] and it was correlated with the intense innervation of the striatum [Wicht and Himstedt, 1988; González et al., 1994]. Since double labeling for the simultaneous detection of catecholaminergic markers and CB and CR has not been conducted in Dermophis, we can not rule out colocalization in some of the ventromedially located cells. Again, without double-labeling techniques, the dopaminergic mesencephalic cell groups of mammals were reported to contain numerous CRir [Résibois and Rogers, 1992] and CBir cells [Celio, 1990; McRitchie et al., 1996; Verney et al., 2001].
Isthmus
The isthmus, as considered here (r0), is a thin and oblique segment in other amphibians, but in gymnophionans the curvature is more accentuated. The sharp curve of the rostrocaudal axis and the large development of the dorsal mesencephalon cause an irregular obliquity of this boundary, and actual isthmic nuclei were classically included in the mesencephalic or in the rhombencephalic tegmentum. In the alar part of the isthmus, the isthmic nucleus as identified by its cholinergic cells [González et al., 2002a] is very reduced, like most visual centers in gymnophionans [Himstedt and Manteuffel, 1985; Roth et al., 1993], and no CBir or CRir cells are located in a similar position. This observation is in agreement with the lack of CB and CR in the homologous nucleus of other amphibians and other vertebrate groups [Celio, 1990; Díaz-Regueira and Anadón, 2000; Castro et al., 2006; Morona and González, 2009]. Like in anurans and urodeles, the isthmic tegmentum of Dermophis shows CBir and CRir cells as caudal continuations of the mesencephalic groups (Pdi and Pvi). In turn, the trochlear nucleus, characteristic of the basal tegmentum [González et al., 2002a], seems to be devoid of CB and CR. It is important to note that only 1% of the trochlear motoneurons in the cat contain CB [De la Cruz et al., 1998] and no data are available in other vertebrates.
Hindbrain
In accordance with previous studies in amphibians [Marín et al., 1997; Straka et al., 2006; Morona and González, 2009] we consider the isthmo-rhombencephalic boundary as described above and the rhombencephalon starting rostrally in the large segment r1. Many derivatives have been described in r1 that are distinct enough to have received an anatomical name. This is especially observed in those derivatives of the alar plate that include cholinergic cell groups, the locus coeruleus, the parabrachial complex, the main sensory trigeminal nucleus, part of the rostral octaval column, and lateral parts of the corpus cerebelli and the auriculae [Aroca and Puelles, 2005]. Moreover, the tegmentum is characterized by the absence of motoneurons and the presence of the interpeduncular nucleus. By means of CB and CR immunohistochemistry, we could distinguish some of those components in the r1 of Dermophis and analyze their correct topological localization.
In the alar part of r1, a cholinergic and nitrergic cell group was described previously in the ‘isthmic’ region misplacing the boundaries [González et al., 2002a, b]. Comparing the distribution of the cholinergic and nitrergic cells with the CBir and CRir cell populations [own observations], most of the cholinergic cells actually belong to r1, and they most likely correspond to the LDT, as defined in other vertebrates [Aroca and Puelles, 2005]. Among the cells of this nucleus, a minor subpopulation most likely colocalizes CB or CR, as found in other amphibians [Morona and González, 2009] and mammals [Nemcová et al., 1997; Fortin and Parent, 1999].
Another r1 derivative is the locus coeruleus. This noradrenergic group was described next to the LDT in the ‘isthmic’ region [González and Smeets, 1994]. CB and CR cells are located in a similar position in Dermophis; however, no double labeling was performed to corroborate the colocalization. In other amphibians, double-labeling experiments for CB and CR with tyrosine hydroxylase corroborated that the locus coeruleus neurons do not possess CB or CR [Morona and González, 2009]. CBir and CRir cells are also present along the alar plate of r1 in Dermophis in topological positions similar to where the octavolateral nuclei, the parabrachial nucleus (area), and the main sensory trigeminal nucleus were described in anurans and urodeles [Morona and González, 2009].
Many studies in amphibians and other vertebrates corroborated the presence of CB and CR in different cerebellar cell types. Specifically, CB is a good marker of Purkinje cells [Fortin et al., 1998; Schmidt et al., 2005, 2007], whereas CR is found mainly in other cell types as granular [Gall et al., 2003], unipolar, brush, and Lugaro cells [Résibois and Rogers, 1992; Schiffmann et al., 1999], except in primates where some subpopulations of Purkinje cells are CRir [Fortin et al., 1998]. In all species of amphibians studied so far CB is present in Purkinje cells and some granule cells [Uray et al., 1998; Uray and Gona, 1999; Morona and González, 2009] and particularly in Rana but not in Xenopus laevis, and urodeles Purkinje cells contain CR [Necchi et al., 1999; Uray and Gona, 1999; Morona and González, 2009]. However, the cerebellum of gymnophionans is so poorly developed that several studies have suggested that it might be absent altogether [Fritzsch and Sontag, 1987; Roth et al., 1993]. In Dermophis we observed a small population of CBir cells with long processes that because of its position might be regarded as a plausible candidate for a minimal cerebellum in these animals.
In the basal region of r1, the interpeduncular nucleus and neuropil of Dermophis contain noticeable CBir fibers originated in the dorsal habenula and coursing in the fasciculus retroflexus, like in urodeles [Morona and González, 2008, 2009]. The particularly intense staining for CB and CR in segregated populations of the central gray cells is a shared feature observed in the r1 of other amphibians and teleosts [Díaz-Regueira and Anadón, 2000; Castro et al., 2006; Morona and González, 2009].
Along the basal part of the rhombencephalon, a column of CBir and CRir cells is present in Dermophis, as in other vertebrates. Its extent across rhombomeres can be assessed by localization of the motor nuclei and nerve roots [González et al., 2002a]. Most likely, many of these cells correspond to reticulospinal cells [Sánchez-Camacho et al., 2001], as demonstrated in other vertebrates [Goodchild et al., 2000; Morona et al., 2006b, 2007a]. Also in the basal part of the rhombencephalon, comparison of the distribution of CBir and CRir cells with the location and size of the motoneurons in the trigeminal and facial nuclei strongly support colocalization, although it was not corroborated by double-labeling experiments. In addition, as proposed for the other orders of amphibians, the efferent cells of the octaval nerve could also be CBir or CRir in Dermophis according to their position [Fritzsch and Crapon de Caprona, 1984].
Throughout the rhombencephalon, in the dorsal part of the alar plate, mixed populations of CBir and CRir are present in the undifferentiated octaval column of Dermophis [Fritzsch, 1988]. This situation contrasts with that observed in anurans and urodeles because, in those species that retain the lateral line system in the adult, CR is particularly related to the mechanoreceptive lateral line cells and primary fibers, whereas CB is mainly present in octaval cells and fibers. In addition, the anurans that have lost the lateral line system through metamorphosis show CR immunoreactivity mainly in acoustic fibers and nuclei, whereas CB is mainly present in vestibular fibers and nuclei [Morona and González, 2009]. As previously noted, adult Dermophis most likely do not posses a lateral line system and the present results suggest that the vestibular and acoustic information are not well segregated in the rhombencephalic alar plate.
Caudally in the alar plate of Dermophis, distinct labeling for CB and CR was found in the nucleus of the solitary tract and the dorsal column nucleus, as defined by their content in cholinergic, catecholaminergic, and nitrergic cells [González and Smeets, 1994; González et al., 2002a, b]. This feature seems to be shared by all amphibians and concurs with data reported in mammals [Crockett et al., 1996; Magnusson et al., 1996; Kawai and Semba, 1999; Morona and González, 2009].
Spinal Cord
The abundant CBir and CRir spinal cells revealed in Dermophis are distinctly distributed in the dorsal and ventral gray fields largely resembling the situation found in urodeles and lungfish [Morona and González, 2009; Morona et al., 2010]. Significantly, in anurans the distribution of CB and CR in the spinal cord includes a variety of cells with different localizations and morphologies in close resemblance with the distributions reported in amniotes [Morona and González, 2006a].
Concluding Remarks
The present study has corroborated that the distribution of CB and CR observed in D. mexicanus conforms to the overall amphibian pattern, but it has also demonstrated that within the class Amphibia remarkable differences exist. It should also be noted that, as was observed for anurans and urodeles, we can not characterize gymnophionans based on work on a single species because important species differences may exist in the distribution of these CBPs. It also becomes clear that before referring to specific ‘amphibian’ patterns of brain organization one should include data from the much neglected group of amphibians, the gymnophionans.
The immunohistochemical technique for detection of CB and CR used in our study was confirmed as a powerful tool in the identification of cell groups and brain structures that are otherwise indistinct in the brain of Dermophis. In addition, the consistent paradigm considered for comparing brain regions has served to clarify, in many cases, the principal criterion for the determination of homology, i.e. similarity in position [Nieuwenhuys, 1998]. However, particularly in gymnophionans, the identification of many neuronal condensations or nuclei cannot be made cytoarchitectonically due to restricted cell segregation from the periventricular region [Roth et al., 1993]. Therefore, immunocytochemically identified neuron populations compared across species represent a useful auxiliary tool that gives important clues in relation to the homology of brain nuclei [Nieuwenhuys, 1998]. However, it should be pointed out that straightforward comparisons based only on the presence of CBPs should not be made, due to the great variability observed in well-established homologous regions in the brain of different vertebrates (see above). Nevertheless, in cases of unclear topological relations, this immunohistochemical approach has been valuable to confirm homology [Dávila et al., 2000; Milán and Puelles, 2000; Puelles and Rubenstein, 2003; Morona and González, 2008, 2009].
Acknowledgements
We are grateful to Prof. R.G. Northcutt (Scripps Institution of Oceanography, UCSD) for his help in obtaining some specimens of D. mexicanus. This research was supported by the Spanish Ministry of Science and Technology (grant No. BFU2009-1231/BFI).



![Fig. 1. Scheme of a lateral view of the brain of D. mexicanus in which the revised and simplified prosomeric model has been adapted [Puelles and Rubenstein, 2003]. There are 3 segments in the diencephalon, i.e. prosomeres 1–3. The rostralmost forebrain contains the basal and alar parts of the hypothalamus. Following this model, the hypothalamus is divided into rostral and caudal transverse subdomains. It has also been subdivided into separate areas in the alar and basal parts: the preoptic (PO), SPV, suprachiasmatic (SC), paraventricular (PV), tuberal (Tub), and mammillary (Ma) regions. The telencephalon represents an extension of the alar hypothalamic region and is further subdivided into the pallium and subpallium. The segmentation of the brainstem follows that described previously [González et al., 2002a]. The a–z and a–w levels of the sections shown in figures 2 and 3 are indicated.](https://karger.silverchair-cdn.com/karger/content_public/journal/bbe/77/4/10.1159_000329521/2/m_000329521_f01.gif?Expires=1716429628&Signature=uHZqIYUdJTaT8c3hDqDXfwrOW7dj03ydqxzI4MjFuLodJBSrg~VTx-eHL4ir0ox85a0mp1JPZUfHjhuGFMfP11RNuRqiI2QBQE2TY0TQlHdboemYFFNW9tXAPaGbcSclkRbGzxFtPgBh~E-0fbbwFq5j0-wr5~ooR2~lFY9xQqxUfIVxeYEmiTspT99VUQ1O7RDdZCXo9UME9LnW209SHUerMlMtH5GrWcNG3~klsl-xWaMDbhHG9GtQ4OXsDx3mF1ooV6OsrTtaVD1-zDytcaYYRzSSenR5g-d~D~ivunlDLHr3~Z4icOYTTLH~~YfIjGWZtR170EsTIPjumsPDuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)