Abstract
Background: Patients, especially young children, with atopic dermatitis are at an increased risk of developing eczema vaccinatum, a severe reaction to the smallpox vaccine, either through direct vaccination or indirect contact with a person recently vaccinated. Methods: Using a mouse model of infection, the severity of vaccinia-induced lesions was assessed from their appearance and viral DNA content. The response to vaccinia inoculation was assessed in young and adult mice, allergen-sensitized mice, and in mast cell-deficient mice. Results: Young age, sensitization to an allergen prior to infection, and a mast cell deficit, accomplished by using mast cell-deficient mice, resulted in more severe viral lesions at the site of inoculation, according to lesion appearance and viral DNA content. All three factors combined demonstrated maximal susceptibility, characterized by the severity of primary lesions and the development of secondary (satellite) lesions, as occurs in eczema vaccinatum in humans. Resistance to the appearance of satellite lesions could be restored by adoptive transfer of bone marrow-derived mast cells from either wild-type or cathelicidin-related antimicrobial peptide-deficient mice. Primary lesions were more severe following the latter transfer, indicating that cathelicidin-related antimicrobial peptide does contribute to the protective activity of mast cells against infection. Conclusions: The combination of young age, allergen sensitization and a mast cell deficit resulted in the most severe lesions, including satellite lesions. Understanding the factors determining the relative resistance/sensitivity to vaccinia virus will aid in the development of strategies for preventing and treating adverse reactions which can occur after smallpox vaccination.
Introduction
Smallpox is a potentially deadly disease, killing approximately 30% of those infected by the virus [1]. It was successfully eradicated, with the last known case occurring in Somalia in 1977 [2]. However, due to the terrorist events of September 11, 2001, there was rising concern over the use of smallpox as a biological weapon [3]. This led to the reintroduction of the smallpox vaccination, specifically among first-line responders such as medical and military personnel [4,5].
Vaccination with vaccinia virus (VV) is effective in protecting against smallpox but is contraindicated in patients with atopic dermatitis (AD) due to a potentially lethal side effect, eczema vaccinatum (EV) [6,7]. EV is characterized by a disseminated response to VV with lesions spreading outside of the initial vaccination site. Most often this type of reaction is limited to the skin, but in some cases the reaction may be systemic with infection of internal organs. EV is most commonly seen among children aged 1–5 years old, suggesting that the age of the host is a major determinant of outcome [8,9,10]. It is unclear why this severe, life-threatening reaction to smallpox vaccination is primarily limited to this group of allergic, young patients.
AD is a chronic skin disorder that affects nearly 20% of children in the USA [11,12]. The diagnosis of the disease is complex, and several immunological defects are thought to predispose AD patients to EV including, but not limited to, decreased cytotoxic T cell generation, reduced IFN-γ production, increased Th2 cell responses, and reduced natural killer (NK) T cell function. Also, a lack of neutrophils but an increased number of mast cells in the skin and insufficient skin levels of antimicrobial peptides, such as LL-37 and β-defensins which have potent antiviral activity against VV, are mechanisms that have been suggested [13,14,15,16,17,18,19,20,21,22]. Given these characteristics of AD patients – raised serum IgE levels, early age of onset, and more mast cells in the skin – we hypothesized that these three factors play important roles in the response to VV. Cultured bone marrow-derived mast cells (BMMCs) from mice have been shown to produce cathelicidin-related antimicrobial peptide (CRAMP) [23], the murine homolog of LL-37 which can provide protection against VV [17]. On the other hand, the high serum IgE levels seen in AD may stimulate mast cells to secrete IL-4 and IL-13 and inhibit CRAMP production [24]. Thus, mast cells could have a dual role, one which is protective, the other enhancing susceptibility, depending on predisposing conditions in the host. Here, susceptibility or resistance to VV was determined in different strains of mice in order to define the contributions of age, mast cells and allergic sensitization. The most severe infection, characterized by satellite lesions outside of the initial inoculation site as seen in EV, only occurred when all three factors were combined, i.e. young age, allergen sensitization and a lack of mast cell function in the host.
Materials and Methods
Virus and Cells
The virus stock was generated from the ATCC VR-1354 Western Reserve strain of VV. It was grown in HeLa S3 cells (American Type Culture Collection, Manassas, Va., USA) and titered according to the procedures described previously [17,25].
Mouse Strains
Adult Balb/c mice (10 weeks of age) were obtained from the Jackson Laboratory (Bar Harbor, Me., USA) and adult C57BL/6 mice from Harlan Laboratories (Indianapolis, Ind., USA). Mast cell-deficient KitW-sh/W-sh mice [26,27] were obtained from Dr. M. Maurer (Charité, Berlin, Germany). CRAMP-deficient mice on the C57BL/6 background were kindly provided by Dr. D.Y.M. Leung (National Jewish Health, Denver, Colo., USA) and had been prepared by breeding with the CRAMP-deficient Cnlp–/– mouse strain [28] obtained from Dr. R.L. Gallo (University of California, San Diego, Calif., USA). Young mice (4 weeks old) were bred on site. Breeding and experimental mouse protocols used in this study were approved by the Institutional Animal Care and Use Committee of National Jewish Health.
Intraperitoneal Sensitization and VV Infection
Mice were sensitized with 20 µg ovalbumin (OVA; Calbiochem, La Jolla, Calif., USA) in alum, intraperitoneally, at 2 and 3 weeks of age. Intraperitoneal sensitization was chosen because it resulted in a rapid elevation of IgE levels in all animals and could be easily accomplished with young mice prior to virus infection. At the age of 4 weeks, mice were inoculated with VV. Prior to inoculation, mice were shaved and then treated briefly with Nair (Church and Dwight Inc., Princeton, N.J., USA) 2 days prior to inoculation. Animals were anesthetized and then inoculated by applying 1 × 107 plaque-forming units of VV to the dorsal skin and scratching 20 times with a bifurcated needle [17]. Mice were observed for 7 days and then sacrificed by CO2 asphyxiation. Skin specimens from primary lesions, uninvolved skin and satellite lesions were obtained using 4-mm-punch biopsies. Blood was obtained by cardiac exsanguination.
Determination of OVA-IgE
High-binding ELISA plates were used to quantitate OVA-specific IgE [29].
Mast Cell Culture and Mast Cell Transfer
Bone marrow cells were prepared from mouse femurs (C57BL/6 or CRAMP–/– mice) and grown to yield mast cells, as described previously [30]. The medium used to grow and induce differentiation of mast cells contained purified recombinant IL-3 (15 ng/ml; Biosource, Camarillo, Calif., USA) and stem cell factor (25 ng/ml; Biosource). After 4 weeks in culture, homogeneous cultures of BMMCs were confirmed, as described previously [31], and were used for experiments at 4–6 weeks after initiation of the culture. At 1 week prior to inoculation with VV, 5 × 106 mast cells were injected intraperitoneally into the KitW-sh/W-sh mast cell-deficient strain of mice.
Real-Time PCR for Detection of VV DNA
Genomic DNA was isolated from the skin samples using the Qiagen DNeasy Blood and Tissue Kit (Valencia, Calif., USA). Real-time PCR was performed for the detection of VV DNA using 200 ng of genomic DNA, 900 nM primers and 200 nM probe specific for vaccinia ribonucleotide reductase [32] (Applied Biosystems, Foster City, Calif., USA) and 2× Taqman Universal PCR Master Mix (Applied Biosystems) in a 25-µl reaction volume. The amount of viral DNA in skin samples was calculated using a standard curve generated with known amounts of VV DNA subjected to the same PCR conditions. VV DNA amounts were normalized to the amount of input genomic DNA per reaction.
Histological Analysis of Skin
Skin biopsies were fixed in 10% formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin or with Astra Blue for the visualization of mast cells.
Statistical Analysis
Data are expressed as means ± SEM. The unpaired, 2-tailed t test, performed using GraphPad Prism, was used to determine differences between the 2 groups. The p values for significance were set to 0.05.
Results
Age as a Factor in VV Susceptibility
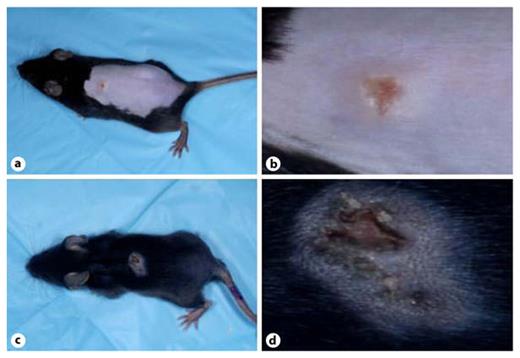
In mice, age has been shown to be critical in virus susceptibility, especially in neonates [33,34]. To test the effect of age on susceptibility to VV infection, we compared responses in mice inoculated at 4 weeks of age to those inoculated at 10 weeks. As shown in figure 1, the primary lesions of young Balb/c mice (fig. 1c, d) were more severe than those of adults (fig. 1a, b), with less healing and a greater exudate. The more severe lesions of young mice also appeared to be larger in size. However, lesion size was not quantitated because it was also dependent on the area of the initial inoculation site, which was difficult to control since infection was performed by multiple scratchings with a bifurcated needle, as already described. Of more importance, VV DNA in lesions was determined as a quantitative measure of VV susceptibility and lesion severity. It was found that young Balb/c mice showed significantly more viral DNA in their primary lesions than their adult counterparts (fig. 1e) in this animal model suggesting that young age indeed enhances susceptibility to VV.
Young age increases susceptibility to VV in Balb/c mice. Severity of primary lesion appearance in 10-week-old mice (a, b) was compared to that of 4-week-old mice (c, d) at 5 days after inoculation with VV. The 4-week-old mice had lesions that had healed less and showed greater exudates than the lesions of the 10-week-old mice. e When assayed at 7 days after infection, the primary lesions of 4-week-old Balb/c mice contained more VV DNA than their 10-week-old counterparts (n = 6 for each group). * p = 0.02.
Young age increases susceptibility to VV in Balb/c mice. Severity of primary lesion appearance in 10-week-old mice (a, b) was compared to that of 4-week-old mice (c, d) at 5 days after inoculation with VV. The 4-week-old mice had lesions that had healed less and showed greater exudates than the lesions of the 10-week-old mice. e When assayed at 7 days after infection, the primary lesions of 4-week-old Balb/c mice contained more VV DNA than their 10-week-old counterparts (n = 6 for each group). * p = 0.02.
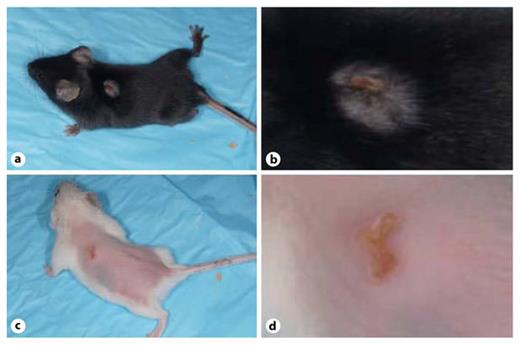
Similar results were found with the C57BL/6 strain of mice. As shown in figure 2, lesions on young mice (fig. 2c, d) appeared to be more severe than on adults (fig. 2a, b). As with the Balb/c strain, they showed less healing, a greater exudate and more apparent inflammation. Young C57BL/6 mice also showed significantly more viral DNA in their primary lesions than their adult counterparts (fig. 2e). In these experiments, satellite lesions, indicative of virus spread beyond the site of inoculation, were not seen in any adult or young mice.
Young age increases susceptibility to VV in C57BL/6 mice. Severity of primary lesion appearance in 10-week-old mice (a, b) was compared to that of 4-week-old mice (c, d) at 5 days after inoculation with VV. The 4-week-old mice had lesions that had healed less and showed greater exudates than the lesions of the 10-week-old mice. e When assayed at 7 days after infection, the primary lesions of 4-week-old C57BL/6 mice contained more VV DNA than their 10-week-old counterparts (n = 7 for each group). * p = 0.04.
Young age increases susceptibility to VV in C57BL/6 mice. Severity of primary lesion appearance in 10-week-old mice (a, b) was compared to that of 4-week-old mice (c, d) at 5 days after inoculation with VV. The 4-week-old mice had lesions that had healed less and showed greater exudates than the lesions of the 10-week-old mice. e When assayed at 7 days after infection, the primary lesions of 4-week-old C57BL/6 mice contained more VV DNA than their 10-week-old counterparts (n = 7 for each group). * p = 0.04.
Mast Cells as a Factor in VV Susceptibility
Mast cell infiltration occurs in the skin of those affected with AD [22], but it is unclear whether this is protective or increases susceptibility to virus. The response to VV infection was compared between two strains of mice, the mast cell-deficient strain mice, KitW-sh/W-sh and their age-matched, 4-week-old, wild-type (WT) counterparts. Upon visual inspection, the severity of the lesions did not appear dramatically different between the KitW-sh/W-sh mice (fig. 3c, d) and the WT C57BL/6 mice (fig. 3a, b). However, when DNA content of the primary lesions was assessed, the mast cell-deficient KitW-sh/W-sh mice showed significantly more viral DNA in their lesions than the WT mice (fig. 3e). These findings suggested that at least under these isolated conditions, mast cells play a protective role against VV infection.
Lack of mast cells increases susceptibility to VV, shown by comparison of viral DNA content in primary lesions of young KitW-sh/W-shmast cell-deficient and young C57BL/6 WT mice. Visual inspection showed no dramatic differences between the primary lesions of the WT, 4-week-old C57BL/6 mice (a, b) and the 4-week-old, mast cell-deficient, KitW-sh/W-sh mice (c, d) at 5 days after inoculation with VV. e However, at 7 days after infection, the primary lesions of the 4-week-old KitW-sh/W-sh mice (n = 9) contained more VV DNA than the primary lesions of the 4-week-old C57BL/6 mice (n = 10). * p = 0.01.
Lack of mast cells increases susceptibility to VV, shown by comparison of viral DNA content in primary lesions of young KitW-sh/W-shmast cell-deficient and young C57BL/6 WT mice. Visual inspection showed no dramatic differences between the primary lesions of the WT, 4-week-old C57BL/6 mice (a, b) and the 4-week-old, mast cell-deficient, KitW-sh/W-sh mice (c, d) at 5 days after inoculation with VV. e However, at 7 days after infection, the primary lesions of the 4-week-old KitW-sh/W-sh mice (n = 9) contained more VV DNA than the primary lesions of the 4-week-old C57BL/6 mice (n = 10). * p = 0.01.
Allergic Sensitization as a Factor in VV Susceptibility
Patients with AD have raised serum IgE levels and overexpress the Th2 cytokines, IL-4 and IL-13, known inhibitory factors of virus containment, in their skin [16,24]. Hence, allergic sensitization could render the host more susceptible to VV infection. The experiments described above were performed using nonsensitized mice. Next, experiments were performed to compare 4-week-old Balb/c mice which had or had not been systemically sensitized according to the protocol described in the Materials and Methods section. Sensitization was confirmed by demonstrating OVA-specific IgE in the serum (data not shown).
No substantial differences were detected in overall primary lesion appearance between the sensitized and nonsensitized groups (data not shown). However, when the viral DNA amounts in the primary lesions were quantified for the Balb/c strain of mice, a dramatic difference was found (online suppl. fig. 1A; for all online suppl. material, see www.karger.com?doi=10.1159/000330647). Similar experiments were performed comparing sensitized and nonsensitized young KitW-sh/W-sh(C57BL/6) mice. A significant increase in the amount of viral DNA was seen in the sensitized mice of this strain as well (online suppl. fig. 1B). These increases indicated that allergen sensitization did, in fact, enhance VV susceptibility to virus infection and/or replication. Results obtained with the mast cell-deficient strain suggested the possibility that sensitization might further enhance the increased susceptibility already conferred by a mast cell deficit.
Combined Effect of Age, Mast Cell Status, and Sensitization in Susceptibility to VV
To test the notion that a combination of the factors might be especially detrimental to the host, we compared the course of infection in old and young allergen-sensitized, KitW-sh/W-shmice. As demonstrated in figure 4, the primary lesions of the adult sensitized Ki tW-sh/W-sh mice were limited to the sites of inoculation (fig. 4a, b). In contrast, the primary lesions of the young sensitized KitW-sh/W-sh mice appeared far more severe with the lesions being more exudative, and, of most importance, spread outside of the initial inoculation site (fig. 4c, d). The appearance of these multiple secondary or satellite lesions was limited to the young group, where all of the mice developed satellite lesions compared to none amongst the adults. Also of importance, analysis of viral DNA content showed that the primary lesions from the sensitized young group contained significantly more viral DNA than detected in the sensitized mast cell-deficient adult mice (fig. 4e). These results indicated that the combination of all three factors renders the host most susceptible to VV. Of most importance, multiple satellite lesions were seen only in young, sensitized, mast cell-deficient mice.
Combination of young age, sensitization and mast cell deficit increases susceptibility to VV infection, indicated by severity of primary lesions, satellite lesions and viral DNA content. Five days after infection, primary lesions of the adult (10-week-old), sensitized KitW-sh/W-sh mice were limited to the site of inoculation (a, b) while primary lesions of the young (4-week-old) mice appeared more severe, with spreading outside of the inoculation site (c, d). Satellite lesions (arrows) were seen in all young mice compared to no satellite lesions in the adult mice. e Seven days after infection, the primary lesions of the young, sensitized KitW-sh/W-sh mice (n = 13) contained more VV DNA than was found in lesions from the adult, sensitized KitW-sh/W-sh group (n = 7). * p = 0.04.
Combination of young age, sensitization and mast cell deficit increases susceptibility to VV infection, indicated by severity of primary lesions, satellite lesions and viral DNA content. Five days after infection, primary lesions of the adult (10-week-old), sensitized KitW-sh/W-sh mice were limited to the site of inoculation (a, b) while primary lesions of the young (4-week-old) mice appeared more severe, with spreading outside of the inoculation site (c, d). Satellite lesions (arrows) were seen in all young mice compared to no satellite lesions in the adult mice. e Seven days after infection, the primary lesions of the young, sensitized KitW-sh/W-sh mice (n = 13) contained more VV DNA than was found in lesions from the adult, sensitized KitW-sh/W-sh group (n = 7). * p = 0.04.
Protective Effects of Transferred Mast Cells
In light of the potential dual role of mast cells in the response to VV, we examined the role of mast cells in the most susceptible hosts, using a mast cell transfer protocol. Mast cells from C57BL/6 (WT) mice were adoptively transferred into young allergen-sensitized KitW-sh/W-sh mice with the goal of restoring ‘resistance’ to VV, comparable to that observed in the mast cell-sufficient WT strain. In addition, mast cells from CRAMP–/– mice (on the C57BL/6 background) were adoptively transferred into young sensitized KitW-sh/W-shmice to assess whether the absence of mast cell CRAMP would have an effect on virus susceptibility. Mast cells were transferred intraperitoneally 7 days before virus inoculation. Successful transfer and appearance of mast cells in the skin was confirmed by Astra Blue staining of skin biopsies 2 weeks later (fig. 5f, i).
Reconstitution of resistance to VV infection following adoptive transfer of BMMCs into mast cell-deficient mice. Mast cells from either C57BL/6 (d, e) or CRAMP-deficient (Cnlp–/–) mice (g, h) were adoptively transferred to young, sensitized KitW-sh/W-shmice. Lesions appeared worse in the KitW-sh/W-shmice that did not receive mast cells (a, b) with infection forming satellite lesions. Lesions from mice that received BMMCs from Cnlp–/– mice (g, h) appeared worse than those that received BMMCs from C57BL/6 WT mice (d, e). Successful mast cell transfer was confirmed by Astra Blue staining of skin biopsies (f, i). No mast cells were detected in KitW-sh/W-sh mice that did not receive mast cells (c). The mice and tissue shown are representative of experiments with KitW-sh/W-shmice that did not receive mast cells (n = 16), KitW-sh/W-shmice which received WT mast cells (n = 19) and KitW-sh/W-shmice which received Cnlp–/– mast cells (n = 14). c, f, i ×200.
Reconstitution of resistance to VV infection following adoptive transfer of BMMCs into mast cell-deficient mice. Mast cells from either C57BL/6 (d, e) or CRAMP-deficient (Cnlp–/–) mice (g, h) were adoptively transferred to young, sensitized KitW-sh/W-shmice. Lesions appeared worse in the KitW-sh/W-shmice that did not receive mast cells (a, b) with infection forming satellite lesions. Lesions from mice that received BMMCs from Cnlp–/– mice (g, h) appeared worse than those that received BMMCs from C57BL/6 WT mice (d, e). Successful mast cell transfer was confirmed by Astra Blue staining of skin biopsies (f, i). No mast cells were detected in KitW-sh/W-sh mice that did not receive mast cells (c). The mice and tissue shown are representative of experiments with KitW-sh/W-shmice that did not receive mast cells (n = 16), KitW-sh/W-shmice which received WT mast cells (n = 19) and KitW-sh/W-shmice which received Cnlp–/– mast cells (n = 14). c, f, i ×200.
As expected, the young sensitized KitW-sh/W-shmice that received no mast cells had the most severe-looking lesions as well as the development of satellite lesions (fig. 5a, b). Following adoptive transfer of C57BL/6 mast cells into young sensitized KitW-sh/W-sh mice, primary lesions were observed but satellite lesions were not seen in this group (fig. 5d, e), indicating that mast cells can indeed provide protection against the virus. Adoptive transfer of CRAMP-deficient mast cells into young sensitized KitW-sh/W-shmice was also effective in eliminating the development of secondary lesions (fig. 5g, h). Of major importance, secondary lesions were not seen on any animals which received transferred mast cells of either type, indicating the importance of these cells in limiting the spread of the virus beyond the site of inoculation, a key determining factor in EV. The primary lesions in mice reconstituted with CRAMP-deficient mast cells did appear (fig. 5g, h) more severe than those observed in the group that received WT mast cells (fig. 5d, e), indicating that mast cell-derived CRAMP may, in part, contribute to the protective activity of mast cells against VV infection, a notion supported by the histological analysis of skin sections, as described below.
Histological analysis of skin sections showed marked differences in the degree of inflammation as well as in the type of cellular infiltrate found between the mast cell-deficient and reconstituted groups (fig. 6). An uninfected skin sample from a KitW-sh/W-sh mouse was characterized by well-defined layers of epidermis, dermis, subcutaneous fat and muscle (fig. 6a). In contrast, ulceration with massive cellular infiltration and inflammation into the subcutaneous fat and muscle layers was observed after infection with VV in mast cell-deficient skin (fig. 6b). The cellular infiltrate in this group was mainly comprised of mononuclear cells with some plasma cells found in submuscular areas (fig. 6b inset). Following mast cell reconstitution with WT mast cells, inflammation was clearly reduced and was more superficial with less involvement of the fat and muscle layers (fig. 6c). It was also notable that epidermis was normal with little inflammation in sections from mast cell-reconstituted skin. In contrast, skin from the mice receiving mast cells from the CRAMP–/– mice was also less inflamed than the mast cell-deficient group but appeared more inflamed than the group that received WT mast cells, as indicated by a greater degree of involvement in the deep tissue (fat and muscle) layers (fig. 6d). The cellular infiltrate in both mast cell-reconstituted groups was found to be mostly polymorphonuclear cells (insets in fig. 6c, d), suggesting that at least one of the protective roles of mast cells against VV is through the recruitment of neutrophils to the site of infection. As neutrophils are a major source of antimicrobial peptides [35], these observations may explain, at least in part, why both CRAMP-sufficient and -deficient mast cells can play a protective role against VV infection following adoptive transfer into young sensitized KitW-sh/W-sh mice.
Histopathology of skin adjacent to primary VV-induced lesions. a The appearance of the skin in uninfected KitW-sh/W-sh mice. Dense cellular infiltrates were seen in young sensitized mast cell-deficient infected KitW-sh/W-shanimals (b), which were greatly reduced following adoptive transfer of BMMCs from WT C57BL/6 animals (c), and much less so following the transfer of BMMCs from CRAMP–/– animals (d). b–d insets Cellular composition of the infiltrates. The sections are stained with H&E stain. The tissue sections are representative of experiments with KitW-sh/W-shmice that did not receive mast cells (n = 16), KitW-sh/W-shmice which received WT mast cells (n = 19) and KitW-sh/W-shmice which received Cnlp–/– CRAMP–/– mast cells (n = 14). a–d ×100. Insets ×1,000.
Histopathology of skin adjacent to primary VV-induced lesions. a The appearance of the skin in uninfected KitW-sh/W-sh mice. Dense cellular infiltrates were seen in young sensitized mast cell-deficient infected KitW-sh/W-shanimals (b), which were greatly reduced following adoptive transfer of BMMCs from WT C57BL/6 animals (c), and much less so following the transfer of BMMCs from CRAMP–/– animals (d). b–d insets Cellular composition of the infiltrates. The sections are stained with H&E stain. The tissue sections are representative of experiments with KitW-sh/W-shmice that did not receive mast cells (n = 16), KitW-sh/W-shmice which received WT mast cells (n = 19) and KitW-sh/W-shmice which received Cnlp–/– CRAMP–/– mast cells (n = 14). a–d ×100. Insets ×1,000.
Discussion
A number of animal models of EV have been explored by others to understand the biological and molecular basis for the potentially life-threatening VV dissemination which can be seen in some individuals after vaccination against smallpox [36,37,38]. Virus inoculation at sites of experimentally induced Th2-biased allergic skin inflammation resulted in more severe lesions, higher viral DNA loads in the skin and internal organs and more satellite lesions when compared to inoculation on unsensitized skin [36]. Local expression of IL-17 and ineffective T cell responses may also be involved in the susceptibility of patients with AD to EV [36,37,38].
Since EV and other adverse reactions to smallpox vaccination occur most frequently in children [8,9,10], we compared the course of viral infection in ‘young’ (age approx. 4 weeks) and ‘old’ (age approx. 10 weeks) mice. Although primary lesions were considerably more severe and contained higher amounts of viral DNA in young mice, satellite lesions were rarely, if ever, observed in these animals. Studies using herpes simplex virus [33] or respiratory syncytial virus [34 ]have also suggestedthat viral infection might be enhanced in young mice compared to in older animals. Eczema herpiticum, a rare complication of AD resulting from disseminated cutaneous herpes simplex infection, has also been shown to be associated with young age and elevated serum IgE levels [39]. Our report also demonstrated that systemic sensitization to an allergen markedly enhanced susceptibility to VV, as determined by viral DNA amounts in primary lesions.
Mast cell status was also found to contribute to lesion severity and viral DNA content. The findings that mast cells are a source of CRAMP (the rodent equivalent of human LL-37) [23] and that CRAMP could directly inactivate VV [17] also indicate that mast cells play a role in host defense against VV. In addition, mice deficient in the production of CRAMP were more susceptible to viral infection [17], and lesional skin from AD patients was relatively high in IL-4 and IL-13 [40,41,42], cytokines which downregulate AMP production in some cell types [24]. Dissemination of the virus to form satellite lesions was also observed at a higher frequency in Cnlp–/– CRAMP-deficient mice [17].
To investigate the role of mast cells and mast cell-derived CRAMP in protection against VV infection, experiments were performed using the mast cell-deficient KitW-sh/W-sh strain of mice [26,27]. These mice were more susceptible to infection than age-matched WT mice. However, even with young mast cell-deficient animals, dissemination of the virus to form satellite lesions was not seen, but when sensitization was combined with young age and a mast cell deficit, the formation of multiple satellite lesions was routinely observed.
The role of mast cells in protection against VV infection and spread was confirmed by restoring the relative resistance following the adoptive transfer of WT mast cells. In mast cell-reconstituted mice, lesions appeared far less severe and, more importantly, secondary lesions were no longer seen, indicating that mast cells played a role in limiting the spread of the virus beyond primary lesions. Mast cells generated from CRAMP-deficient mice were partially effective in restoring resistance. Differences were also seen in the tissue surrounding the primary lesions, with the inflammation being the most severe in mast cell-deficient mice. The infiltration into deeper layers was more pronounced in animals which received mast cells prepared from CRAMP–/– mice than those from WT mice, suggesting that CRAMP played a role in limiting infection and/or the inflammatory response to VV. Strikingly, mononuclear cells predominated in the severe lesions in animals lacking mast cells, whereas neutrophils were more abundant in the lesions of mast cell-reconstituted animals, suggesting that mast cells were involved in neutrophil recruitment and that this was beneficial to the host. Mast cells are involved in the recruitment of other cell types, including neutrophils, during inflammation [43]. Neutrophils are a potent source of CRAMP [35], more so than mast cells on a cellular basis (unpubl. results), and this could also limit the spread of infection. However, it is proposed that high mast cell numbers without neutrophil recruitment, as seen in AD, may not be sufficient to confer protection. In fact, elevated mast cells alone may be detrimental, since, as noted above, they secrete cytokines which can inhibit antimicrobial peptide production by other cells, such as keratinocytes [24].
In several experiments, it was necessary to compare the development and appearance of lesions in animals with different hair status. For example, it was noted that younger mice clearly regrew hair faster than older mice (fig. 1, 2). Also, mast cell-deficient KitW-sh/W-shmice reconstituted with mast cells routinely showed delayed hair regrowth compared to mice which did not receive the adoptive transfer (fig. 5 and data not shown). Since mice could not be treated with hair-removing agents or reshaven after virus infection, this may have interfered somewhat with the evaluation of lesion appearance. Of more interest, given the possible importance of the immune system, especially of mast cells, throughout the hair follicle cycle [44,45,46], differences in the cycle might reflect or even account for differences in lesion appearance or virus spread.
The studies presented here indicate that the degree of reaction to VV inoculation which occurs during vaccination is the result of a combination of numerous factors, including young age, allergen sensitization and mast cell functions. Combined with results implicating T cells, natural killer cells and neutrophils, and the cytokines and other factors which they produce, these results indicate that a complex network of cells and cell products determines the relative resistance/susceptibility to VV infection. Use of the animal models of infection described here will permit further delineation of this network with the goal of designing strategies to limit viral dissemination in susceptible individuals.
Acknowledgements
Masakazu Okamoto and Yoshiki Shiraishi are gratefully acknowledged for their assistance in preparing bone marrow cells. Diana Nabighian provided expert assistance in the preparation of the manuscript. This research was supported by the NIH/NIAID (HHSN266200400030C, ADB Contract N01-AI-40030). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.