Abstract
The development of ventricular assist devices (VADs) over the past 5 decades as therapy for advanced heart failure (HF) has been extraordinary. Since the original VAD design by Michael DeBakey in the early 1960s, numerous devices for mechanical circulatory support have been engineered, assessed in preclinical studies, applied to human patients in large multicenter clinical trials, and now, select devices are Food and Drug Administration-approved therapy for advanced HF patients. This review highlights select examples of durable VADs from the engineering aspect of design and conception to experimental studies and clinical application underscoring the remarkable progression of such technology to now becoming the standard of care for many advanced HF patients.
Introduction
Heart failure (HF) is a worldwide pandemic. According to the latest American Heart Association Heart Disease and Stroke Statistics 2011 Update, HF affects approximately 5.7 million Americans with an incidence of 670,000 new HF cases ≥45 years of age [1]. It is estimated that HF affects more than 23 million people worldwide [2]. The gold standard for the treatment of end-stage HF is heart transplantation. However, the glaring discrepancy between the number of available heart donors and the growing population of end-stage HF patients requires other treatment options for this advanced HF population, in particular, mechanical circulatory support (MCS) with a left ventricular assist device (LVAD). In the US, it is estimated that the number of theoretical adult candidates ≥20 years of age with advanced American Heart Association/American College of Cardiology stage C and D HF eligible for LVAD therapy is in the range of 100,000-300,000 [3].
The MCS era began with the founding of the Artificial Heart Program in 1964 at the National Institutes of Health. Michael DeBakey was the first to develop the original rudimentary LVAD prototypes and, in 1966, reported the first successful use of the LVAD in a young woman unable to be weaned from cardiopulmonary bypass. Since that time, the era of MCS ushered in the development of various support devices that entered laboratory and preclinical testing to improve on the initial LVAD designs. In 1994, the Food and Drug Administration (FDA) approved the pneumatically driven LVAD as bridge to transplant (BTT) and, in 1998, approved the self-contained, vented electric LVAD devices for the same indication. These first-generation LVADs have been shown to improve end-organ function, optimize hemodynamics, augment functional capacity and improve the quality of life (QOL) of end-stage HF patients awaiting heart transplantation with an acceptably low side effect profile [4, 5, 6, 7, 8, 9]. Figure 1 shows a representative first-generation LVAD with the device implanted in the abdominal cavity below the diaphragm, with the inflow cannula positioned in the left ventricular apex drawing blood from the left ventricle and the outflow cannula anastomosed to the ascending aorta [10]. There is a driveline cable that exits the skin and is attached to an external controller that is powered by portable batteries. This first-generation device is now essentially obsolete.
Representative diagram of a first-generation LVAD positioned in the human body. Adapted with permission from Rose et al. [10].
Representative diagram of a first-generation LVAD positioned in the human body. Adapted with permission from Rose et al. [10].
The landmark REMATCH trial (Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure) assessed the outcomes of end-stage HF patients who were ineligible for heart transplantation that were randomized to medical management or implantation of the HeartMate vented electric LVAD (Thoratec Corporation, Pleasanton, Calif., USA) [10]. The investigators demonstrated a marked reduction of 48% in the risk of death from any cause in the group randomized to the LVAD arm with a 1-year survival rate of 52% in the device group compared to 25% in the medical therapy group. However, despite these results, this technology was not initially widely embraced by the medical community due to concerns regarding the large size of the pump, limited durability of the device and adverse events such as infections related to the large percutaneous driveline.
Over the past 15 years, the second generation of pumps utilizing continuous-flow (CF) technology has been developed with only a single moving part, the rotor, thus significantly reducing the size of the pump and providing more durable support with less mechanical wear compared to the first-generation LVADs. Recently, third-generation pumps without mechanical bearings have been developed that suspend the impeller with magnetic or hydrodynamic suspension systems. The CF rotary pump design consists of axial (mainly second-generation) or centrifugal (mainly third-generation) pump platforms. The main differences between axial and centrifugal pump performance may best be illustrated by analyzing the differences in pump head curves of the two designs. The pump head curve describes the relationship between the flow generated by an axial or centrifugal VAD and the difference in pressure across the inlet and outlet of the pump (pump delta P) that is generated as the VAD and is placed in between the left ventricle and systemic circulation. There is a separate pump head curve that may be generated with different pump speeds. With axial flow pumps, there is a steep and inverse linear relationship between flow and pump delta P. For example, flow increases as pump delta P decreases and vice versa. If the pump delta P is 40 mm Hg in ventricular systole and 80 mm Hg in diastole, for axial flow pumps, this produces a less pulsatile waveform ranging from 3 to 7 l/min during the cardiac cycle (fig. 2) [11].
Pump head curves of a centrifugal pump and axial flow pump and the effects on flow and pulsatility waveforms. Adapted with permission from Pagani et al. [11].
Pump head curves of a centrifugal pump and axial flow pump and the effects on flow and pulsatility waveforms. Adapted with permission from Pagani et al. [11].
In contrast, centrifugal pumps have a relatively flat head curve whereby these pumps operate over a wide range of flows for small changes in delta P across the pump. With the same delta P as illustrated above of 40 mm Hg in systole and 80 mm Hg in diastole, the centrifugal pump generates large changes in flow ranging from 0 to 10 l/min, similar to a pulsatile LVAD (fig. 2) [11].
Another important difference between axial and centrifugal pump designs is the performance of the pump as flow decreases. As illustrated in figure 3[11], as pump flow decreases from 4 to 2 l/min (which may happen clinically with dehydration, arrhythmias or right ventricular failure), there is a significant increase in pump delta P which may predispose to inlet cannula obstructive events, known as ‘suction events'. With centrifugal pump designs, as the pump flow drops from 4 to 2 l/min, the delta P remains constant, thus inlet suction events would be less common.
Differences in pump delta P of an axial flow pump and a centrifugal pump with changes in speed and flow. Adapted with permission from Pagani et al. [11].
Differences in pump delta P of an axial flow pump and a centrifugal pump with changes in speed and flow. Adapted with permission from Pagani et al. [11].
In 1998, the MicroMed-Debakey VAD was the first of four axial CF pump designs to enter the clinical arena, followed by the Jarvik 2000, HeartMate II (HM II) and Incor [12, 13, 14, 15]. The first centrifugal magnetically levitated platform to enter clinical trials was the Terumo DuraHeart in 2004 [16]. A simplified schematic of a volume displacement pump, axial flow pump and centrifugal designs is shown in figure 4[17]. Because of the smaller size, increased durability and decreased risk of infection due to a smaller driveline, the newer-generation CF LVADs are the most commonly used devices today. The recent Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) report, a registry of FDA-approved durable devices jointly sponsored by the National Heart, Lung, and Blood Institute (NHLBI), Centers for Medicare and Medicaid Services (CMS), the FDA and industry, clearly demonstrates the trend in the use of CF LVADs compared to the first-generation pulsatile pumps (fig. 5) [18] and the predominance of univentricular left-sided support compared to the total artificial heart (TAH) or biventricular support (fig. 6) [18]. In this review, we will focus on the development and clinical application of select second- and third-generation CF pumps as these devices represent the most commonly used mechanical assist device technology today (table 1).
Simplified schematic of LVAD designs: volume-displacement pump, axial flow pump and centrifugal pump. Adapted with permission from Baughman and Jarcho [17].
Simplified schematic of LVAD designs: volume-displacement pump, axial flow pump and centrifugal pump. Adapted with permission from Baughman and Jarcho [17].
Primary LVAD implantation as reported in the INTERMACS registry from June 23, 2006, to June 30, 2011, stratified by year of implant and device type. LVAD = Left ventricular assist device; TAH = total artificial heart. Adapted with permission from Kirklin et al. [18]
Primary LVAD implantation as reported in the INTERMACS registry from June 23, 2006, to June 30, 2011, stratified by year of implant and device type. LVAD = Left ventricular assist device; TAH = total artificial heart. Adapted with permission from Kirklin et al. [18]
Primary LVAD, TAH or Bi-VAD patient enrollment in the INTERMACS database from June 23, 2006 to June 30, 2011, stratified by univentricular or biventricular support and year of implant. Adapted with permission from Kirklin et al. [18].
Primary LVAD, TAH or Bi-VAD patient enrollment in the INTERMACS database from June 23, 2006 to June 30, 2011, stratified by univentricular or biventricular support and year of implant. Adapted with permission from Kirklin et al. [18].
HM II Left Ventricular Assist System
The original design of the HM II left ventricular assist system (LVAS) began in 1989 at Nimbus Medical, Inc. (Rancho Cordova, Calif., USA), which previously demonstrated the feasibility of the small axial flow Hemopump. In 1991, a research partnership was established between the Nimbus Company and University of Pittsburgh's McGowan Center for Organ Engineering. Initial animal studies as early as 1992 have demonstrated that the HM II pump had the capability to generate up to 10 l/min of flow for up to 90 days without significant hemolysis. In 1998, the Nimbus Company was acquired by Thermo Cardiosystems, Inc., where further engineering modifications were made. The original purge system bearing design of the device was derived from the original Hemopump and was later revised to journal bearings and subsequently to the ball-and-socket hydrodynamic bearing design. On July 27, 2000, the first human implantation of the HM II device occurred in a 64-year-old Israeli man at the Chaim Sheba Medical Center in Tel Hashomer, Israel. In 2001, the pump technology was acquired by Thoratec Corporation.
The initial experience of feasibility and safety of the HM II device stemmed from the European experience that revealed the thrombotic potential of such devices. In fact, there was clot formation observed near the inlet and outlet stators where blood flow velocity and turbulence are high. Engineers reanalyzed this issue and redesigned the inner lining of the LVAD from the textured surface similar to the first-generation HeartMate XVE to a proprietary smoother lining with less thrombotic potential.
The first report of the implantation of the newly designed HM II device in the United States was by Frazier et al. [14] in November 2003 in an 18-year-old recipient. He went from New York Heart Association (NYHA) class IV symptoms to class I with improvement in end-organ function, underwent physical rehabilitation and was awaiting heart transplantation with more than 6 months of mechanical support at the time of the report.
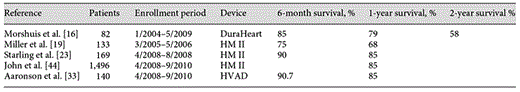
Four years later, Miller et al. [19] published the results of a multicenter study of 133 advanced HF BTT candidates that were implanted with the HM II device. The primary outcome was the proportion of patients who, at 180 days after implant, had a heart transplant, demonstrated heart recovery with explantation of the device, or had continued mechanical support while remaining on the transplant waiting list. The primary outcome was achieved in 100 patients (75%), with survival rates while on support of 75% at 6 months and 68% at 12 months. These improved results heralded a growth of LVADs, and the percentage of patients supported with an LVAD before transplant has increased.
In patients not eligible for transplantation (destination therapy or DT), Slaughter et al. [20] described the outcomes of a randomized trial comparing patients who were implanted with the HM II device compared to the older-generation pulsatile LVAD. In a 2:1 randomized fashion, 134 patients were assigned to receive the CF LVAD and 66 patients were assigned to the pulsatile device. The primary outcome was the composite of, at 2 years following implantation, survival free from disabling stroke and reoperation to repair or replace the LVAD. The primary outcome was achieved in more patients implanted with the CF pump (46%) versus those implanted with the pulsatile device (11%; p < 0.0001). The 1- and 2-year survival rates after implant for the CF pump were 68 and 58%, respectively, compared to the 1- and 2-year postimplant survival rates for those with the pulsatile device, i.e. 55 and 24%, respectively. Park et al. [21] recently published the mid-trial DT results demonstrating improved 1- and 2-year survival rates (73 and 63%, respectively) compared to the early trial outcomes, along with improved side effect profile.
In addition to survival benefit, the CF LVADs significantly improve the functional status and QOL of patients. Data from patients enrolled in the BTT (n = 281) and DT (n = 374) HM II LVAD clinical trials were analyzed to assess changes in functional status (NYHA class, 6-min walk test, patient activity scores) and QOL (Minnesota Living with Heart Failure, Kansas City Cardiomyopathy Questionnaire) before and after LVAD implantation. Rogers et al. [22] report both early and sustained improvements in functional class: 82% of BTT and 80% of DT patients at 6 months and 79% of DT patients at 24 months after implant had improvements in NYHA class from IV at baseline to I or II [20]. The mean 6-min walk test of DT patients at baseline 204 m improved to 350 and 360 m at 6 and 24 months, respectively, after implant. Also, the patient-reported outcomes, QOL as reported by the Minnesota Living with Heart Failure and Kansas City Cardiomyopathy Questionnaire scores, improved.
On April 21, 2008, the FDA approved the HM II device for patients awaiting heart transplantation and on January 20, 2010, for those ineligible for heart transplantation. Using data acquired from the INTERMACS registry, Starling et al. [23] evaluated the first 169 consecutive patients who were implanted with the HM II CF device who were listed for transplantation or likely to be listed from April through August 2008 at 77 centers in the US. The comparison group consisted of patients implanted for the same indication using other FDA-approved mechanical support devices (HeartMate XVE and Implantable Ventricular Assist Device, Thoratec Corporation) during the same time period. At 6 months, the percentage of patients who were transplanted, achieved myocardial recovery or had ongoing LVAD support was 91% for the HM II group and 80% for the comparison group. For those with ongoing LVAD support, the 1-year postimplant survival was 85% for HM II and 70% for the comparison group. These results confirm the earlier results of the HM II BTT pivotal trial [19] in a real-world, commercial, post-FDA approval market setting, lending further support to the continued use of CF LVADs for the treatment of advanced HF as BTT. The post-FDA approval results in the use of such devices as DT is currently being analyzed. To date, more than 10,000 HM II devices have been implanted worldwide and it is projected that this number will continue to increase rapidly.
Jarvik 2000 LVAS
The Jarvik 2000 (Jarvik Heart, Inc., New York, N.Y., USA) is an axial valveless CF pump that has a unique miniaturized design allowing implantation of the device intraventricularly, thus eliminating the need for an inlet cannula and for the surgical creation of a pump pocket below the diaphragm to house the pump. The original design was conceptualized by Robert Jarvik, with the blood pump inserted into the left ventriuclar apex and an outflow graft measuring 16 mm that is anastomosed to the ascending, descending or even the abdominal aorta. Because of the small size (90 g, 2.5 cm in diameter, similar to the size of a C-sized household battery), the device may be implanted via a standard sternotomy or a left thoracotomy approach and may be performed without the need for cardiopulmonary bypass or aortic cross-clamping. The pump speeds are typically in the range of 8,000-12,000 rpm with adjustments of 1,000 rpm increments via an analog controller, with average flow rates of 3-7 l/min. The blood-contacting surfaces of the pump are made of smooth titanium. Initial in vivo results were promising, demonstrating excellent hemocompatibility and short- and mid-term survival outcomes [24]. In addition, for this particular device, the surgeon may choose a postauricular pedestal instead of an abdominal cable as a driveline, which decreases the likelihood of device-related infections.
The US Pivotal BTT trial of 153 patients has completed enrollment and follow-up as of May 2012 with the analysis ongoing. The study received approval for an additional 25-patient enrollment in the Continued Access Protocol. In August 2012, the FDA announced the approval for the Pivotal DT trial RELIVE (Randomized Evaluation of Long-Term Intraventricular VAD Effectiveness).
DuraHeart LVAS
Since the mid 1990s, Terumo Corporation, the NTN Corporation and Setsunan University have been collaborating together to design a centrifugal pump with a magnetically suspended impeller system. Ex vivo and in vivo large animal studies have demonstrated long-term durability, no thrombogenicity and a low hemolysis rate on more than 2 years of support with this device [25]. The DuraHeart LVAS represents the first magnetically levitated centrifugal pump to enter into clinical trials in January 2004, with a total enrollment of 82 patients implanted with the DuraHeart LVAS through May 2009 in Europe. A total of 23 patients (28%) received a heart transplantation while supported on the device with a median time to transplantation of 157 days. The overall Kaplan-Meier survival for patients who continued on device support was 90% at 13 weeks, 85% at 6 months, 79% at 1 year and 58% at 2 years after device implantation. The DuraHeart LVAD is the first third-generation LVAD to be awarded market approval (Conformité Européenne mark) in February 2007 and is currently commercially available in Europe [16]. The Japanese BTT trial completed enrollment with premarket analysis ongoing. Currently, preclinical studies of the DuraHeart II device are underway with first in man implant anticipated for 2013.
VentrAssist LVAS
The VentrAssist LVAS (Ventracor Ltd.) also represents the third-generation LVAD design utilizing a hydrodynamic suspension along with a pump housing design maximizing blood flow to the center of the rotor as well as overlying its outer surface, thus maximizing washout of all blood contacting surfaces and minimizing the potential thrombotic risk. This pump was tested in the animal lab over an approximate 4-year period with a cumulative experience of 55 sheep and a total support time of 4.8 years [26, 27]. The results of the single-arm multicenter BTT clinical trial of 33 patients demonstrated that at the 154-day prespecified postimplant trial end point, 39.4% of patients were transplanted and 42.4% continued on device support with transplant eligible status with a total success rate of 82%, meeting the primary outcome of survival to transplant or continued transplant eligibility while on device support [28]. However, soon after this trial was completed, the company was declared bankrupt and this device is no longer available for clinical use.
Levacor LVAS
The Levacor device (World Heart Corp.) is a third-generation CF pump that combines both passive and active magnetically levitated components to generate flow. The passive mechanism involves permanent magnets and the active mechanism involves an active magnetic coil that works synergistically. The passive system counteracts almost all of the mechanical forces on the rotor, thus decreasing power consumption. The magnetically levitated system elevates the rotor, resulting in complete suspension with no physical contact with the pump housing. Initial results in humans were reported in a feasibility bridge to recovery protocol [29], but further clinical applications of this device were not realized.
HeartWare LVAS
The HeartWare HVAD (HeartWare, Inc., Framingham, Mass., USA) is a small third-generation centrifugal pump with a fully integrated inflow cannula that is surgically implanted intrapericardially, thus eliminating the need for abdominal surgery to create a pump pocket. Initial animal studies of the HeartWare HVAD include Frazier and colleagues at the Texas Heart Institute where the investigators demonstrated excellent hemocompatibility of the device and end-organ effects in 6 healthy sheep [30]. The HeartWare HVAD entered the clinical arena in 2006. The first multicenter trial conducted in Europe and Australia involved 23 BTT patients in 5 centers with actuarial survival after implantation of 91% at 6 months and 86% at 1 year [31]. At the 180-day endpoint, 2 patients underwent successful heart transplantation, 2 patients died while on the device and 19 patients continued on device support after 180 days after implantation. Importantly, due to the intrapericardial placement of the pump without the need to create an abdominal pump pocket, the operation was relatively quick, with a mean time on cardiopulmonary bypass of 67 min (range 21-140). The study was followed by a larger trial of 50 patients conducted in Europe and Australia [32]. At 2 years, 20 (40%) received a heart transplant, 4 (8%) achieved myocardial recovery with device explantation, and 17 (34%) were on continued device support. The actuarial survival after implantation at 6, 12 and 24 months was 90, 84 and 79%, respectively. As a result of data submitted for the first 25 patients of this trial, in January 2009, HeartWare received the Conformité Européenne mark, and the HeartWare HVAD is currently commercially available in the European Union.
In the United States, the ADVANCE pivotal trial was a prospective, multicenter study to evaluate the HeartWare HVAD in BTT patients [33]. The enrollment period was from August 2008 to February 2010 with 140 patients. The treatment group was compared to a control group that was implanted with FDA-approved commercially available LVADs for the same indication, with data derived from INTERMACS. Of note, this landmark study represents the first LVAD study where the control group was accrued concurrently from the INTERMACS registry. This cooperation between government and industry we anticipate will engender future efficient, cost-effective clinical trials of new LVADs. The 180-day survival was 94% and the 360-day survival was 91%. The 30-day postimplant mortality was 1.4%. The Continued Access Protocol for ADVANCE granted by the FDA includes an additional 202 patients, and enrollment is currently underway. On November 20, 2012, the HeartWare HVAD has been FDA approved for use as BTT.
For patients not eligible for heart transplantation in the United States, ENDURANCE is a randomized, controlled, unblinded multicenter clinical trial to evaluate the HeartWare HVAD as DT compared to any FDA-approved LVAD for the same indication. The planned enrollment of 450 patients is now complete and follow-up data analysis is ongoing.
LVADs in Less Sick but Advanced HF Patients
The current CMS criteria for insurance reimbursement regarding LVAD placement for DT are shown in table 2. Traditionally, LVADs have been primarily used to salvage patients presenting in cardiogenic shock or who have advanced end-stage HF; however, the MCS field is moving away, using durable mechanical support devices in these situations due to poor outcomes. Boyle et al. [42] have demonstrated improved survival and reduced hospital length of stay in patients implanted with an LVAD in INTERMACS profiles 4-7 compared to sicker advanced HF patients in INTERMACS profiles 1-3.
Given the advancement of LVAD pump technology with improvement in survival outcomes and the transition to the newer miniaturized CF pumps, the application of such technology to a broader population of HF patients that might benefit needed to be considered. In 2008, a working group consisting of experts in HF cardiology, cardiac surgery, medical ethics and regulatory affairs was convened by the NHLBI to advise on the latest HF management strategies, in particular on the potential application of LVADs to a ‘less ill' but advanced HF population as it relates to clinical trial design [43]. The working group recommended that a multicenter randomized clinical trial comparing LVAD therapy in advanced, ambulatory, non-inotrope-dependent NYHA class IIIB/IV patients versus optimal medical management be performed to address this question. The NHLBI and National Institutes of Health are sponsoring the multicenter REVIVE-IT (Randomized Evaluation of VAD Intervention before Inotropic Therapy) feasibility clinical trial. This prospective, open-labeled, randomized trial will compare the LVAD therapy to optimal medical management in select non-inotrope-dependent patients with advanced HF who are not candidates for heart transplantation.
Thoratec Corporation has launched the ROADMAP (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients) study that is currently enrolling. This is a prospective, multicenter, nonrandomized, controlled observational study evaluating the Thoratec HM II LVAS compared to optimal medical management in NYHA class IIIB or IIIB/IV non-inotrope-dependent patients who are not listed for heart transplantation or are not planned for such listing in the following 12 months of enrollment.
Both trials will be provocative and informative regarding the use of LVADs in ambulatory advanced HF patients and may expand current CMS indications for MCS.
Limitations of LVADs
Despite the remarkable development of LVADs from conception, preclinical studies, large randomized clinical trials now to FDA-approved therapy for advanced HF, there are important limitations of LVAD therapy that need to be considered. The percutaneous driveline that exits the skin may potentially serve as a nidus of infection, in particular if the driveline site is not cared for adequately, or if the driveline itself is accidently pulled due to physical trauma. In addition, CF LVADs require both antiplatelet and anticoagulation therapy that will exacerbate gastrointestinal bleeding, in the setting of LVAD-acquired von Willebrand's syndrome or arteriovenous malformation. Conversely, cerebrovascular events (hemorrhagic, ischemic) may occur, sometimes despite therapeutic levels of anticoagulation. Although uncommon, one of the most feared and potentially life-threatening complications is pump thrombosis that may require treatment with intensified intravenous anticoagulation/antiplatelet regimens, thrombolytic therapy or pump exchange. The development of de novo aortic insufficiency (AI) has been described with LVAD therapy due to decreased left ventricular end-diastolic pressures as the left ventricle is unloaded combined with increased aortic root pressure due to the output of the device. Also, AI may develop with fusion of the aortic valve leaflets if the speed of the device is maintained at high speeds for prolonged periods of time. If severe, AI would cause a vicious loop of decompensated HF as the LVAD would not be able to fully unload the ventricle. To help minimize the development of significant AI following LVAD implantation, it has become customary to repair the aortic valve at the time of LVAD implant if AI is at least of moderate degree and to maintain aortic valve opening at a 1:3 ratio to the cardiac cycle. Finally, it is an area of intense investigation regarding the potential long-term effects of CF physiology on end-organ function. John et al. [44] described the complete side effect profile of 1,496 HM II patients in the HM II BTT post-trial era where the most common adverse events associated with HM II implantation were bleeding (38%) and infection (36%). Similarly, a review of the INTERMACS database between June 2006 and December 2011 has demonstrated that among 1,160 CF LVADs that were implanted as DT, the highest adverse event rates/100 patient-months in the first year after implantation was bleeding and infection, followed by respiratory failure, neurological events and renal dysfunction [45].
Future Directions of MCS and Clinical Recommendations
The adverse events mentioned above underscore the need for continual research and development for future LVAD designs to continue to improve morbidity and mortality as well as patient satisfaction and QOL. Designs are underway for smaller third-generation CF pumps that would allow for faster and easier surgical implantation without compromising durability and flow generation. Rechargeable batteries with prolonged battery life span as well as improving peripheral equipment to make them smaller and lightweight to increase patient comfort are being developed. One of the Achilles' heels of current LVAS is the percutaneous driveline. There are developments underway to design completely implantable LVADs with internal components, including implantable batteries that are rechargeable transcutaneously. Such a system would allow an LVAD patient to be completely submerged in water and participate in activities such as swimming and would importantly eliminate driveline-related infections completely. Remote monitoring and device interrogation akin to the advances seen in defibrillators and cardiac resynchronization therapy devices are future goals of MCS therapy.
Many valuable insights to guide the future of MCS therapy have been learned from the INTERMACS registry (www.intermacs.org). Improved LVAD outcomes and access has fueled marked growth in the utilization of MCS therapy in the US and globally. Perhaps the most important lesson learned from INTERMACS is that the sickest patients (INTERMACS level 1) have the highest mortality. The awareness of this relationship has prompted a shift in utilization with a decrease in implants in INTERMACS 1 patients and greater rates in INTERMACS level 2 and 3 [18]. The additional major ‘shift' has been an increased use in patients awaiting cardiac transplantation and a reduction in the waiting list mortality since 2006 [46]. The heart transplant community now embraces the philosophy that contemporary LVAD therapy used ‘earlier' rather than as a bail out strategy for a critically ill patient provides improved survival and QOL. LVADs now can increasingly allow patients with advanced HF the opportunity to live longer with better QOL outside the hospital during the lengthy wait for a donor organ.
Conclusion
The evolution of MCS over the past 5 decades has been extraordinary, with the survival outcomes of the most recent BTT and DT LVAD trials summarized in tables 3 and 4, respectively. From the original LVAD prototype designed by Michael DeBakey in the 1960s to the now second- and third-generation CF pumps with marked improvements in patient outcomes and device durability/capability, these are the results of a multidisciplinary approach of advancing the field of MCS from conception to in vitro and animal models to now becoming the standard of care for many advanced HF patients. With the ever-increasing worldwide epidemic of HF and the advances of LVAD therapy on morbidity, mortality, functional status and QOL, MCS will remain a cornerstone in the arsenal of therapeutic options in the care of the advanced HF patient.
Recent survival outcomes (6 months, 1 year and 2 years after LVAD implantation) in recent LVAD BTT trials
Recent survival outcomes (1 year and 2 years after LVAD implantation) in recent LVAD DT trials




![Representative diagram of a first-generation LVAD positioned in the human body. Adapted with permission from Rose et al. [10].](https://karger.silverchair-cdn.com/karger/content_public/journal/crd/125/1/10.1159_000346865/5/m_000346865_f01.jpeg?Expires=1716439553&Signature=uP3Y~N8p8ejRT9Z88xkP8qM2igebV6Jk4pDypFTa~-HSIpa5sKiZccRqK3j-vzTnCexjsNVG70xOGVCm3yS8y1QwcnSk3TFeRhgfTEI1Mf0juAuiT-PzWlmkn0AkgKqWkABv3UCpD3Hlu1lG9crydP2rxfSYhv-Gw-kRmcUzuKYv8qoDb~Up-V11GmuyQYcbBUG2MSgtQACpbmQ0BO8of5xkXr2B6cC-9XoN5zerQQAhTn1qP88FtyUjrdlNXbOMsJmSBLMB-x3s0RO~46YpriwD0e0FKJJCOdjqCn3HgNOeFKGdp82q3kActdw7dhP1M6IRqcyYSOjKVKMr4vdEKg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Pump head curves of a centrifugal pump and axial flow pump and the effects on flow and pulsatility waveforms. Adapted with permission from Pagani et al. [11].](https://karger.silverchair-cdn.com/karger/content_public/journal/crd/125/1/10.1159_000346865/5/m_000346865_f02.gif?Expires=1716439553&Signature=hOK9yC1O188pnQH6SgLVf18WrM9Y8Nv1FmJZU~EqZIPwak1hle2-AsqgIQSXY6IJer9RefhbODUtJn5JE02f12KsccDf5woDf3coiOmug4LKjBcts0f-xg5LJgjNNRw-EFYd92BSooUY~YgY6NQrqWYEaaX~5YP3mPzbw4JonBhqiRbqpigHAB8QX00Cjk53ID2Kah6yZj1yLbqahJec5JPD2SrUCsgNWuLJbNC4IN~EWUXJmhjxjAF5sZU~Zk2LnZYhRjBIkmV3fUsBh~J9T-TdZybS~7fQ-lvTpWkXbt7WUdyLJ0KM4MYLj~DcDj~tj9ejmanDfC~pNTv6Th30oQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Differences in pump delta P of an axial flow pump and a centrifugal pump with changes in speed and flow. Adapted with permission from Pagani et al. [11].](https://karger.silverchair-cdn.com/karger/content_public/journal/crd/125/1/10.1159_000346865/5/m_000346865_f03.gif?Expires=1716439553&Signature=oLYRZOKHlEZluF7Fm~tJOa1mOoQzpntgT0O2SXIsYewaFE7c-S8kOuBv7X0q8iGCQy-dpboWU3e1a6G6Ve1lfj0DRSUzA9mf8CiIVwzf31c4jMwIa-3cZ6RqGABaZMSBdoJ4wKEjNDWszaj9DJ02qCPe3Q8PrP20ycfrq~nOml428xfd5yklGVIAPsvt5j2uHWm5aBMbNyQJE~RHR~cMvKD48Ubx26PU8kmTBmDlU1bahprf0930kXS92tCTaG~17HMwyAtieDEF1lMPuvwVXK9NjGodmzroZPU1rvsoy2VJ1oyM28mteHpkveA4iRnceERoSLTF24J7GmK4IV~FBg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Simplified schematic of LVAD designs: volume-displacement pump, axial flow pump and centrifugal pump. Adapted with permission from Baughman and Jarcho [17].](https://karger.silverchair-cdn.com/karger/content_public/journal/crd/125/1/10.1159_000346865/5/m_000346865_f04.jpeg?Expires=1716439553&Signature=0BwUGWYFHcRh5pz-mhr6dWgXoJErmsorKO7D5XG2inYTIpBoIAqhkNvTf~eeY6YlZau8tl2YknA7pb4bo1YGfhpiCDMc2dB3qm3NoqqoW0Jbs2CVRitI7Ma1YNsljLFcctp9rVDKD3B1PBNVJiaXZNW8l1~lkLbw~S854DOQW5XO9~rieA504os9GL6xFEUC0sPh6AEQT-vywEIVwsZY0VMNH7~RlgLf1Od3akJtRrD4tOrNghuWC7dkoyHCBHqd7jZZHMGpW-jCJZgGNI39H4XOMspwfFU6eak2EdpEz~igpYSwh0UhAzPxFiQctIkPTG7X6LAcOCv8771pGWziAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Primary LVAD implantation as reported in the INTERMACS registry from June 23, 2006, to June 30, 2011, stratified by year of implant and device type. LVAD = Left ventricular assist device; TAH = total artificial heart. Adapted with permission from Kirklin et al. [18]](https://karger.silverchair-cdn.com/karger/content_public/journal/crd/125/1/10.1159_000346865/5/m_000346865_f05.jpeg?Expires=1716439553&Signature=q1xmcGcanFuDVp~e0a46X-L5hiRd~b9kNxR5JvIgRnWcWQRi2iWkDEBFeNSuBq7YiTTxvdZkb7nepnmios382l3G3X4J1D4JaAZIfIEFPECD0nzdklDOLGAoRYkisRjhQxTuMckdj2t1hJBPkKUWXNwNsfV2hwKYT6McmOiHCd3MGmNTCRTN2XElF62KRiPBX6-jJ0TzAcK5tQy1DOWkHAeOwQ-AuZRPbqG6h9xVKS8eIcouZ3QIJuc8FiEt~GbR5QS40McUubCuDIFlTUz~~c8IGCNMwdstsIuL-9rAFVWXqEvUOEDhAAr8ECdsJVg656TVSmhIJRRWdCc2Mh4Jtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Primary LVAD, TAH or Bi-VAD patient enrollment in the INTERMACS database from June 23, 2006 to June 30, 2011, stratified by univentricular or biventricular support and year of implant. Adapted with permission from Kirklin et al. [18].](https://karger.silverchair-cdn.com/karger/content_public/journal/crd/125/1/10.1159_000346865/5/m_000346865_f06.jpeg?Expires=1716439553&Signature=aRrPOA0-Wu-RDvB1zmSADQWa8rbaMM8VITYl41KNAg1YmjhNAsIshJWtCh-xushLKOKfknqNCjK7H31gGcsiOpqo53Rf1sOhi6VI6JlJNeKoJAbsR1KHUpvobmLqiPSsjSTwj6A97Zo1BSctLwsiENwDRZk~XyA~MlHl3XwT2m2DKcNqO8ihjxHsNK~obJv9fk5dhdw0MhQVMVkIc~9bGFE77DcsKN-CbphhGp0PUAbTckdvEuBqOPCFInWsrBsyefwbS6MDjq81~noQ0XGJtwQCIVfxP9LGJKenofPGnAqTmB6m8SDBk4F5G9TVIT9hYY6BSE~3KMA-6xr1MCj5aA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
