Abstract
Background: Stroke-associated pneumonia (SAP) has been implicated in the morbidity, mortality and increased medical cost after acute ischemic stroke. The annual cost of SAP during hospitalization in the United States approaches USD 459 million. The incidence and prognosis of SAP among intensive care unit (ICU) patients have not been thoroughly investigated. We reviewed the pathophysiology, microbiology, incidence, risk factors, outcomes and prophylaxis of SAP with special attention to ICU studies. Methods: To determine the incidence, risk factors and prognosis of acute SAP, PubMed was searched using the terms ‘pneumonia' AND ‘neurology intensive unit' and the MeSH terms ‘stroke' AND ‘pneumonia'. Non-English literature, case reports and chronic SAP studies were excluded. Studies were classified into 5 categories according to the setting they were performed in: neurological intensive care units (NICUs), medical intensive care units (MICUs), stroke units, mixed studies combining more than one setting or when the settings were not specified and rehabilitation studies. Results: The incidences of SAP in the following settings were: NICUs 4.1-56.6%, MICUs 17-50%, stroke units 3.9-44%, mixed studies 3.9-23.8% and rehabilitation 3.2-11%. The majority of NICU and MICU studies were heterogeneous including different neurovascular diseases, which partly explains the wide range of SAP incidence. The higher incidence in the majority of ICU studies compared to stroke units or acute floor studies is likely explained by the presence of mechanical ventilation, higher stroke severity causing higher rates of aspiration and stroke-induced immunodepression among ICU patients. The short-term mortality of SAP was increased among the mixed and stroke unit studies ranging between 10.1 and 37.3%. SAP was associated with worse functional outcome in the majority of stroke unit and floor studies. Mortality was less consistent among NICU and MICU studies. This difference could be due to the heterogeneity of ICU studies and the effect of small sample size or other independent risk factors for mortality such as the larger neurological deficit, mechanical ventilation, and age, which may simultaneously increase the risk of SAP and mortality confounding the outcomes of SAP itself. The pathophysiology of SAP is likely explained by aspiration combined with stroke-induced immunodepression through complex humeral and neural pathways that include the hypothalamic-pituitary-adrenal axis, parasympathetic and sympathetic systems. Conclusions: A unified definition of SAP, strict inclusion criteria, and the presence of a long-term follow-up need to be applied to the future prospective studies to better identify the incidence and prognosis of SAP, especially among ICU patients.
Introduction
Stroke is a major cause of morbidity and mortality [1,2]. Medical and neurological complications, including pneumonia, are found to be major causes of death after stroke [3]. Post-stroke pneumonia also increases the financial burden on the medical system. The annual cost of this complication approaches USD 459 million [4] and the average marginal cost of hospitalization is USD 27,633 [5]. These factors reflect the importance of preventing this complication. Major advances have been reached in elucidating the pathophysiological mechanisms of stroke-associated pneumonia (SAP), especially the stroke-induced immunodepression [6,7]. In addition, neurological intensive care units (NICUs) have been rapidly expanding in the last few years as, compared to the past, many large ischemic stroke patients are now being managed in specialized NICUs. This may be beneficial in terms of incidence and outcomes of stroke-related complications [8,9]. To our knowledge, the incidence and outcomes of SAP after acute ischemic stroke (AIS) have not been thoroughly reviewed among NICU studies. We reviewed studies on the incidence, risk factors, outcomes and prophylaxis of inpatient acute SAP related to AIS according to the study setting with special attention to studies performed in the NICUs. We divided the studies into 5 categories including NICUs, medical intensive care units (MICUs), stroke units, mixed studies combining more than one setting and rehabilitation studies to see if there is any difference according to the study setting. We also reviewed the mechanisms involved in the pathophysiology of SAP including stroke-induced immunodepression which is likely to play a larger role in intensive care unit (ICU) patients where the stroke severity is higher.
Methods
To determine the incidence, risk factors, microbiology and outcomes of inpatient acute SAP related to AIS, PubMed was searched using the MeSH database for the terms ‘stroke' AND ‘pneumonia' from January 1, 1978 till September 30, 2012. PubMed was also searched using the terms ‘neurology intensive unit' AND ‘pneumonia' to include further NICU studies during the same period, as many are heterogeneous and include more than one specific diagnosis. This resulted in 352 papers. Exclusion criteria included non-English literature, animal studies, case reports, papers that only included intracerebral hemorrhage or subarachnoid hemorrhage without AIS data and chronic SAP papers that studied patients after more than 30 days from stroke onset. Thus, studies in long-term facilities and outpatient settings were excluded. Rehabilitation studies were excluded unless the mean for rehabilitation admission was less than 30 days from stroke onset. This left 54 papers for the final review.
To determine the pathophysiology of stroke-induced immunodepression, we searched the literature in English through PubMed using the term ‘stroke-induced immunodepression' for relevant articles in addition to the data extracted from the above search.
Definitions and General Terms
Post-stroke pneumonia has been used to describe pneumonia that occurs early after stroke [10]. The term ‘stroke-associated pneumonia' has been used for the first time by Hilker et al. [11] referring to this concept. SAP is described as early when it happens in the first 72 h of admission to the hospital. Another classification in use divides SAP into acute (when pneumonia develops within a month of stroke) and chronic (when it occurs later than a month) [10]. Clinical SAP studies used a wide range of criteria to define SAP starting from the Center of Disease Control and Prevention criteria to investigating ventilator-associated pneumonia after stroke to reviewing patients' diagnosis from the charts [1,5,12]. We will use the Center of Disease Control and Prevention criteria to define nosocomial pneumonia [13], as they are the most widely used ones among the studies [14,15]. According to these criteria, health care-associated pneumonia is classified into 3 categories: clinically defined pneumonia, pneumonia with common bacterial or filamentous fungal pathogens and specific laboratory findings and pneumonia in immunocompromised patients. We will mention the first 2 categories since they are more often related to SAP. Clinically defined pneumonia criteria require the presence of a new and persistent infiltrate or consolidation on at least 1 chest X-ray or at least 2 serial chest X-rays in the case of underlying lung disease combined with one of the following clinical signs: fever, leukopenia or leukocytosis and altered mental status in more than 70-year-olds in the absence of other causes. These should be added to 2 of the following signs: new-onset purulent sputum or change in the character of the sputum, new-onset or worsening cough, rales, and worsening of gas exchange. Pneumonia with common bacterial pathogen is defined similarly with a positive culture from the blood, pleural fluid, quantitative culture from the bronchoalveolar lavage, or lung parenchyma. However, only one of the last signs (such as new-onset purulent sputum or new-onset cough) is needed instead of two. Ventilator-associated pneumonia is defined as pneumonia in patients who had a device to control breathing within the 48-hour period before the onset of infection [13].
The Pathophysiology of SAP: The Traditional Aspiration Theory and the Newly Described Stroke-Induced Immunodepression
Aspiration Theory
Traditionally, SAP is thought to be secondary to aspiration [10,16,17]. Aspiration and its related risk factors such as impaired level of consciousness and dysphagia have been found to be important risk factors for SAP across different clinical studies [11,17,18]. Many stroke patients have impaired swallowing mechanisms leading to aspiration of oral content during sleep, which may be theoretically related to abnormal dopamine transmission [10]. Experimental evidence for this phenomenon can be found in guinea pigs by blocking D1 dopamine receptors resulting in inhibition of the swallowing reflex and a decrease in substance P in the end organs [19]. A low substance P level in the sputum was also found in elderly patients with aspiration pneumonia [20] and an increase in the serum level of substance P was observed in a clinical study after treating stroke patients with angiotensin-converting enzyme inhibitor with concomitant resolution of aspiration suggesting a further role of low substance P in aspiration [21]. However, the higher incidence of pneumonia in stroke patients compared to others who suffer from dysphagia or a compromised level of consciousness [22,23], as well as the predominance of infection in the acute stroke phase when the maximum neurological deficit is present [24,25] suggest that another mechanism is involved in SAP pathogenesis inducing an immunological alteration [26].
Stroke-Induced Immunodepression
As early as 1974, immunodepression has been thought to accompany AIS [27]. Since then many advances have been achieved elucidating the specific pathophysiological mechanisms. The immunodepression following stroke is the result of activation of 3 systems: the sympathetic system, the parasympathetic system and the hypothalamic-pituitary-adrenal axis [6,7,28].
In 2003, Prass et al. [29] provided experimental evidence for neuroendocrine-mediated systemic immunodepression after stroke in a mouse model. Their work showed that stroke induced an apoptotoic loss of lymphocytes, a shift from T-helper (Th) 1 to Th2 cytokine production, spontaneous bacteremia and pneumonia. They also found that blocking the sympathetic activity prevented bacterial infection and the use of propranolol (β-blocker) decreased mortality after stroke. The same group later showed in 2006 that nasal inoculation of 200 colony-forming units of Streptococcus pneumoniae was enough to cause pneumonia in a mouse model of cerebral ischemia while 200,000 colony-forming units were required to cause the same disease in sham animals and again pneumonia was preventable by β-blockade [30]. Currently, there is a large body of experimental and clinical evidence to support peripheral lymphocytopenia and immunological switch from proinflammatory Th1 response to anti-inflammatory Th2 response as a result of sympathetic activation after AIS [31,32,33,34]. Sympathetic activation following AIS appears to play a crucial part in stroke-induced immunodepression [6]. These changes are mostly prominent among patients with large strokes [31,32] and they may have a predilection for the stroke location such as the involvement of the insular cortex [35].
There is also evidence for the parasympathetic system-related and hypothalamic-pituitary-adrenal-axis-related abnormalities following AIS [36,37]. Hypothalamic-pituitary-adrenal axis activation results in glucocorticoid secretion from the adrenal gland. Glucocorticoids are known anti-inflammatories and they may cause apoptosis of human T-lymphocytes [38]. High and low circulating cortisol levels are clinically associated with mortality after stroke [36].
Activation of the autonomic parasympathetic centers results in cholinergic activity which suppresses the peripheral cytokine release through macrophage nicotine receptors. This effect has been demonstrated in the adult male Lewis rats by the electric stimulation of the vagal nerve [37]. The neuroendocrine system and the autonomic centers are synchronized through the paraventricular nucleus of the hypothalamus [26]. Thus, these systems function together to induce an immune alteration after stroke [6,7,28].
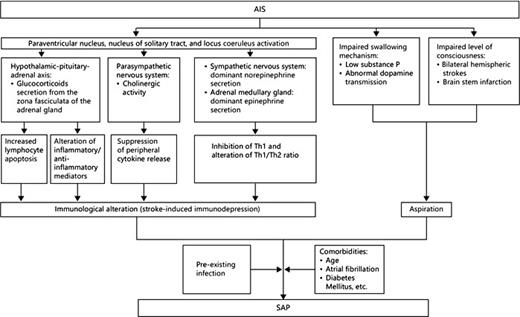
In summary, SAP is likely a net sum of ongoing aspiration which provides the pathogenic bacteria and immunological alterations as part of the stroke-induced immunodepression [7,30]. Various comorbidities add to this equation to increase the risk for SAP (fig. 1).
A scheme demonstrating the pathophysiology of SAP: combining the aspiration theory with the stroke-induced immunodepression theory in addition to the effect of comorbidities.
A scheme demonstrating the pathophysiology of SAP: combining the aspiration theory with the stroke-induced immunodepression theory in addition to the effect of comorbidities.
The Pathophysiology of Potential Stroke Worsening as a Result of Infection
Several mechanisms by which infection or SAP may lead to deterioration of AIS have been proposed. SAP is commonly associated with fever, electrolyte imbalance or hypoxia [26]. These factors may theoretically interfere with the stroke. Fever effects have been extensively investigated in animal models. Fever exacerbates inflammatory cascades resulting in neutrophile accumulation in the injured tissue [39] while therapeutic hypothermia modulates these inflammatory processes [40]. Neuronal excitotoxicity, by increased release of neurotransmitters and free radicals, is another mechanism by which fever may lead to stroke worsening [41]. At the clinical level, a meta-analysis showed that fever is associated with morbidity and mortality after stroke [42]. Electrolyte imbalance, especially hyponatremia, may worsen cerebral edema [43] and hyponatremic stroke patients may have an increased long-term mortality [44]. The entry of bacteria and lipopolysaccharide into the blood stream activates the coagulation as well as fibrinolysis systems and may theoretically result in the extension of the infarcted area [45].
Microbiologic Data
Since aspiration plays an important role in the pathogenesis of SAP, oral and nasopharyngeal pathogens are expected to be encountered frequently [10]. The oral flora of stroke patients is rapidly altered after stroke and aerobic Gram-negative bacilli colonize in the stroke patients' mouths more often than in normal people [46]. Enterobacter sakazakii is another example of organisms that can be isolated from the mouth of stroke patients and are rarely found otherwise [47]. Thus, aerobic Gram-negative bacilli are expected to be the most encountered pathogens. Overall, cultures often remain negative without bacterial growth [15,48]. Staphylococcus aureus which resides in the nasal cavity, and aerobic Gram-negative bacilli including Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii were the most frequently isolated pathogens in the studies identified by our search [11,14,15,23,48,49,50,51]. These findings are consistent with the results of a recent meta-analysis of stroke-associated infections which often showed negative cultures or the same above-isolated pathogens in addition to Escherichia coli and Enterococcus species, while Streptococcus species were occasionally isolated [52]. Thus, the pattern of SAP bacteriology is most consistent with early-onset nosocomial pneumonia or community-acquired aspiration syndrome likely related to aspiration at the time of stroke ictus [52].
Incidence of SAP
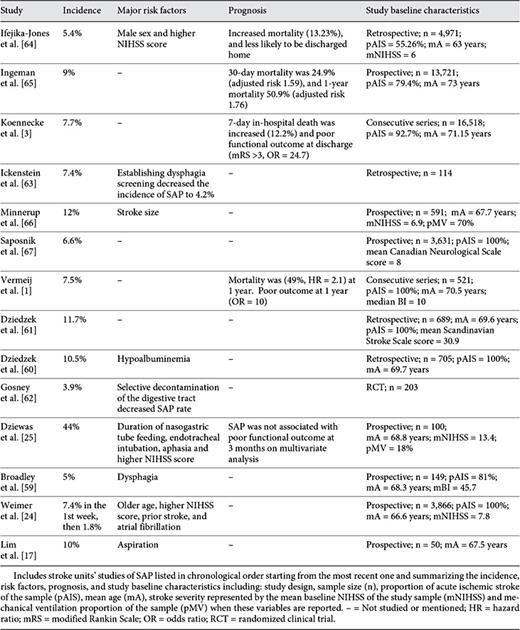
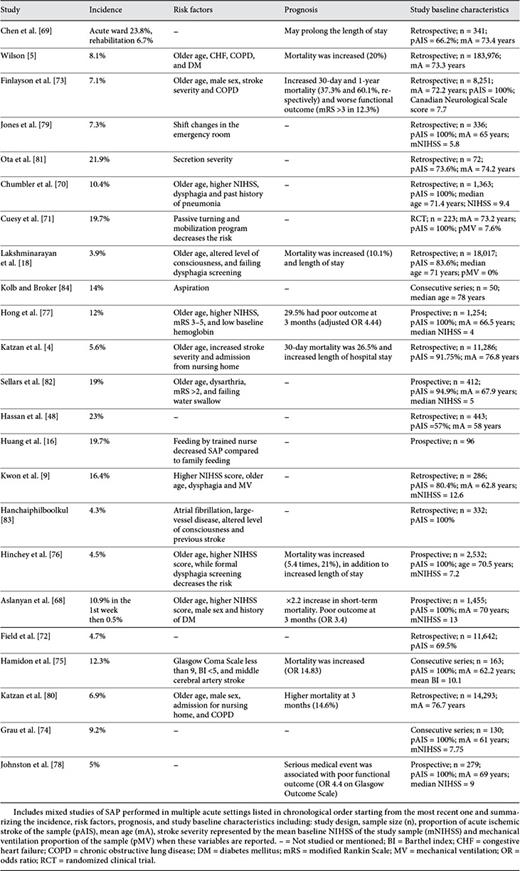
Studies identified by our search methodology are summarized in tables 1, 2, 3, 4, 5. These studies are divided into 5 categories according to the setting in which they were performed: NICUs (table 1), MICUs (table 2), stroke units (table 3), mixed studies (table 4), and rehabilitation units (table 5). Mixed studies include the ones which were performed in more than one acute setting such as the different acute floors, or when the acute settings were not specified.
The incidence of SAP among the majority of NICU studies ranged between 9.5 and 56.6% [11,15,50,51,53,54] except for one study in which the incidence was 4.1% [14]. However, this study included all types of neurovascular patients in addition to stroke patients and they were younger (mean age 58.6 years). The occurrence was much higher among febrile patients (40.2-70.8%) [55,56] reflecting the importance of SAP as a cause of fever after stroke. Among MICU studies, this incidence has ranged between 17 and 50% [12,23,49,57,58] and appeared to be similar to NICU studies. The majority of SAP studies have been performed in stroke units or in mixed acute settings. The incidence of SAP among the majority of studies that have been performed exclusively in stroke units ranged between 3.9 and 12% [1,3,17,24,59,60,61,62,63,64,65,66,67] except for one study where the incidence was 44% [25]. This could reflect a selection bias since all patients included in this study had nasogastric tube feeding, higher rate of mechanical ventilation (18%) and their strokes were more severe [mean baseline National Institutes of Health Stroke Scale (NIHSS) score = 13.4]. Studies performed in mixed acute settings found an incidence between 3.9 and 23.8% [4,5,9,16,18,48,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84], while the incidence among selected rehabilitation studies ranged between 3.2 and 11% [69,85,86,87].
It is hard to make a comparison among these studies because they are highly heterogeneous, especially those performed in critical care settings. The majority of ICU studies included intracerebral hemorrhage or subarachnoid hemorrhage in addition to AIS [14,51,54]. Some have included AIS alone, the majority of which were performed in stroke units or acute general floors [1,11,67,70]. There are also differences in the methods of defining SAP (chart review [64], discharge diagnosis codes [5], or through Center of Disease Control and Prevention criteria [11]), the rates of mechanically ventilated patients vary as well [12,25,66], in addition to the broad geographic distribution of these studies [24,48,50]. Overall, ICU studies are smaller and more heterogeneous compared to floor or stroke unit studies (pooled sample size NICU: 3,756; MICU: 3,145; stroke units: 45,829; mixed studies: 257,232, and rehabilitation: 1,400). The incidence of SAP appears to be similar in NICUs and MICUs and higher than that in the stroke units or acute general floors. These findings are consistent with the results of a recent meta-analysis [52]. This is likely explained by the effect of comorbidities, mechanical ventilation, and higher stroke severity among critical care unit patients causing higher rates of aspiration and immunological alteration as part of the stroke-induced immunodepression.
Prognosis of SAP
The outcome of each specific study is listed in tables 1, 2, 3, 4, 5. It is hard to make comparisons for the same reasons mentioned in the incidence section in addition to the large differences in the follow-up period among these studies [1,5,80]. Using different functional scales such as the Barthel Index or the modified Rankin Scale for assessment of the patient's functional outcome may add to this difficulty [25,68]. Overall, the majority of studies performed in the stroke units and acute hospital floors report SAP as an independent risk factor for mortality after AIS [1,64,65,68,73,80]. The short-term mortality at 30 days or time of discharge ranged between 10.1 and 37.3% [3,5,18,64,65,73,77,80], while long-term mortality was 49-60.1% [1,65,73]. SAP was also associated with poor functional outcome in multiple studies [1,3,63,68,73,77,78]. However, this was not the case in one study after correcting for other independent factors which can be explained by the small sample size (n = 100), preselection of patients with dysphagia and nasogastrictube feeding and high rates of mechanically ventilated patients (18%) [25]. On the other hand, the data regarding outcome of SAP in the critical care units are inconsistent. Hilker et al. [11] showed higher in-hospital mortality (26.9%), higher mortality at 14 months of follow-up (35.3%) and worse functional outcome at 14 months. The rest of the identified ICU studies did not associate SAP with mortality [12,14,23,54,57]. This could be due to the heterogeneity of these studies and the effects of selection bias, small sample size [23] and lack of long-term follow-up [12]. However, the effect of other independent risk factors for outcome, such as high NIHSS score [25], fever [88] and mechanical ventilation, needs to be deeply investigated among ICU studies. These factors may simultaneously increase the patient's risk for SAP, mortality and poor functional outcome confounding the outcomes of SAP itself.
Risk Factors for SAP
Risk factors identified in each study are listed in tables 1, 2, 3, 4, 5. Overall, stroke severity measured by the NIHSS or the modified Rankin Scale is a major independent risk factor for SAP [12,15,24,54,64,66,70,77]. Dysphagia and aspiration are other risk factors, which have frequently been reported in multiple studies [9,11,15,17,59], as well as old age [4,18,24,53,54,70,73]. Other risk factors reported by various studies include: mechanical ventilation [9,11,15], APACHE II score/organ failure status [12], male sex [9,64,68], left anterior cerebral artery stroke or size of the lesion more than 1/3 of the middle cerebral artery territory [66], brain stem infarction [11,23], multihemispheric infarction [11], nonlacunar basal ganglia infarct [15], atrial fibrillation [24,83], admission from a nursing home [80], dysarthria [9,82], altered level of consciousness, coma or abnormal pupillary exam [15,18,80]. Diabetes or hyperglycemia on admission, congestive heart failure, chronic obstructive pulmonary disease or smoking, history of pneumonia and low albumin blood level [5,12,68,70,77], and the presence of other types of infection at the time of admission such as urinary tract infection [15] have all been found to increase the risk for SAP.
Prophylaxis of SAP
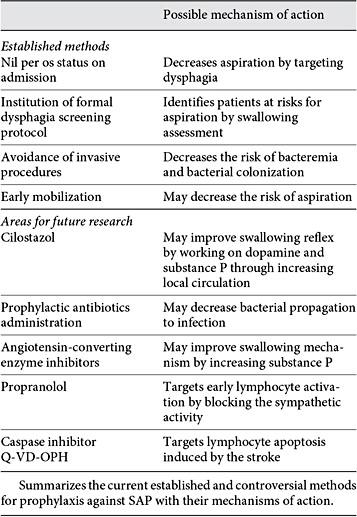
SAP is a complex disease and multiple factors are involved in the pathogenesis as it has been mentioned. Successful prophylactic measures should target these different pathophysiological steps (table 6).
The most widely accepted measure during the management of acute stroke is the nil per os status on admission to avoid overt aspiration till swallowing is checked [89]. The institution of a formal dysphagia screening protocol, including a water swallow test, significantly decreases the risk of pneumonia [63,76]. Enteric feeding methods such as a percutaneous gastrostomy tube or a nasal feeding tube are applied to patients who continue to exhibit signs of unsafe swallowing. These methods do not eliminate the occurrence of pneumonia since aspiration of oral content may continue [25]. A recent Cochrane review of the clinical trials showed no difference between the two methods regarding the occurrence of pneumonia in patients with dysphagia; however, percutaneous gastrostomy tube was safer and more effective in terms of feeding [90]. Early mobilization is recommended as it decreases the risk for SAP [71].
Administration of antibiotics in the acute period following stroke remains a hotly debated topic. Moxifloxacin decreased the incidence of SAP and mortality and improved outcome in an experimental mouse model for stroke [91]. Using this antibiotic in a clinical trial in humans (PANTHERIS) decreased infection rate as per the protocol analysis. However, it failed to show a benefit in the intention-to-treat analysis and it did not affect outcomes or survival [92], and another clinical trial using levofloxacin failed to show a decrease in the infection rate [93]. More recently, the Mannheim Infection in Stroke Study showed that patients in the intervention group, who were administered mezlocillin plus sulbactam over 4 days, had a decrease in body temperature and lower rates of infection, which may be associated with better clinical outcome [94]. The most recently published meta-analysis of clinical trials using prophylactic antibiotics after stroke did show a decrease in the infection rate without any effect on mortality or dependency [95]. To further investigate this matter, the Preventive Antibiotics in Stroke Study, a large randomized clinical trial involving 3,200 patients, has been started. This trial will use 2 g of ceftriaxone as a preventative antibiotic for 4 days. The primary outcomes will be the functional outcomes at 3 months. The rest of the secondary outcomes will involve death at discharge and at 3 months, infection rates during hospitalization and length of stay [96].
Another controversial method is decontamination of the digestive tract in stroke patients aiming to decrease the bacteria colonizing in the mouth and eventually decrease SAP. This method had an effect on SAP incidence in a small study without changing the mortality or morbidity of these patients [62].
Targeting the pathophysiological steps involved in the stroke-induced immunodepression provides an interesting area for research. Propranolol, a β-blocker, has been used experimentally in a mouse model to block the sympathetic activity which induces an apoptotic lymphocytic loss and alteration in the Th1/Th2 ratio. This decreased post-stroke pneumonia and mortality in mice [29]. Another experimental method targets stroke-induced lymphocyte apoptosis in the spleen and thymus by caspase inhibitor Q-VD-OPH. This method improved survival, reduced brain damage, and decreased susceptibility to post-stroke bacteremia in a mouse model [97].
Medications targeting aspiration have been used to decrease the risk of SAP in the chronic stages such as angiotensin-converting enzyme inhibitors which may play a theoretical role in prevention by increasing the level of substance P [21]. Cilostazol, an antiplatelet agent, may theoretically improve the production of substance P and dopamine by improving local circulation [98]. The clinical benefit of both medications was seen among chronic Asian and Japanese stroke patients, respectively, but not in Caucasian patients [98,99,100]. These medications are not likely to help in the acute stages of SAP because of the length of time needed to show the benefit.
Treatment of SAP
SAP treatment must be initiated quickly since SAP may be associated with mortality and worsening of neurological outcome [1]. SAP treatment may follow the guidelines of early hospital-acquired pneumonia therapy [101] since it mainly happens in the first few days after hospitalization [93]. The decision of the empiric antibiotic treatment depends on the individual's risk factors, disease severity, time of onset, general microbiology of SAP and the local microbiologic data of the institution [101].
Conclusions
Despite the major advances that have been achieved in understanding the pathophysiology of SAP in the last few years, there are still a large number of unanswered questions. The true incidence and outcome of SAP, especially in the critical care units, are yet to be determined. The full extent of the stroke-induced immunodepression and its clinical implications are still unknown. Many controversies in the prophylaxis against SAP are present and some clinical trials are on their way. Thus, future research should focus on elucidating the different pathophysiological mechanisms of the stroke-related immunodepression and prospective clinical studies are required to answer the uncertainty of SAP outcomes, incidence and prophylaxis. On the way to achieve that, different heterogeneity factors across the clinical studies must be overcome. Clinical studies need to develop a unified diagnosis of SAP, strict inclusion criteria for stroke patients and differentiation among different stroke subtypes, and they need to have long-term follow-ups with a clear definition of functional outcome, especially among critical care studies. Experimental research should elucidate the rest of the unknown interactions between the brain and immune system which may lead to the discovery of new experimental treatments that may have clinical implications. All of that may lead to developing new treatment and prophylactic measures that aid in limiting this complication which may have detrimental effects on stroke outcomes.
Disclosure Statement
The authors report no conflicts of interest or disclosures.