Abstract
Viruses are the most abundant obligate intracellular entities in our body. Until recently, they were only considered to be pathogens that caused a broad array of pathologies, ranging from mild disease to deaths in the most severe cases. However, recent advances in unbiased mass sequencing techniques as well as increasing epidemiological evidence have indicated that the human body is home to diverse viral species under non-pathological conditions. Despite these studies, the description of the presumably healthy viral flora, i.e. the normal human virome, is still in its infancy regarding viral composition and dynamics. This review summarizes our current knowledge of the human virome under non-pathological conditions.
Humans and Viruses: An ‘I Love You… Me Neither' Story
Since their discovery more than 100 years ago, viruses have been commonly described as obligate intracellular pathogens. Historically, the first studied virus was the one causing rabies, by Louis Pasteur. However, it was the Russian biologist, Dmitri Ivanovsky, and the Dutch botanist, Martinus Willem Beijerinckwent, who first isolated a tobacco-infecting microbe that caused tobacco mosaic disease. Ivanovsky demonstrated that crushed, infected tobacco leaf extracts remained infectious even after Chamberland filtration, which normally retains bacteria. He suggested that the infection might be caused by a bacterial toxin. However, Beijerinck went one step further, concluding that this new pathogen required living plants to replicate and multiply [1]. Subsequent studies showed that viruses infect all domains of life, including bacteria, archaea and eukaryotes, and are found in all ecological niches [2]. This pleiotropic distribution on our planet allows viruses to play the role of ‘natural motors' that drive global energy and nutrient cycling [3,4]. Until very recently, human viruses were considered only pathogens that were capable of causing human pandemics and a wide range of diseases that in some cases lead to a fatal outcome. With the development of new sequencing technologies (see the following section), which have allowed the analysis of the global viral population (DNA and RNA) in humans, known as the human virome, completely new human-associated viruses have emerged [5,6]. However, the majority of these high-throughput sequencing techniques were performed with the use of filters with pore sizes in the range of 0.2-0.45 μm, which filter larger viruses (see the section entitled ‘The human megavirome'), resulting in a technical bias of the human virome. In this context, it became rapidly clear that viral richness and diversity in the human body under non-pathological conditions were widely underestimated. As an example, a rough estimation based on bacteria-infecting viruses (bacteriophages) indicates that there are 100 times more viruses than eukaryotic cells in our body [2,7]. Human-associated viruses control the microbial diversity of the human gut and skin [8,9]. Viruses affect the very foundation of our nature, our genome. Reminiscences of ancestral human-viral cohabitation are imprinted in our genome with approximately 100,000 known endogenous viral fragments, representing approximately 8% of our genome [10]. Finally, endogenous viral proteins have been associated with important physiological functions, such as mammal placental morphogenesis [11,12].
In the present review, we briefly present the evolution of the virological techniques employed in the discovery of human-associated viruses. We then explore existing knowledge of the viral diversity found in human physiological systems under non-pathological conditions. Finally, we discuss the consequences of this human-virus cohabitation.
‘Tracking the Small Guys': Tools for Viral Detection in Humans
Describing the human viral flora requires the right molecular and cellular tools. Historically, classical virology techniques were based on viral isolation from cells and the subsequent observation of cytopathic effects on cell lines or the intracerebral inoculation of suckling mice. Immunological methods, such as seroneutralization or hemagglutination, were then used to detect viral antigens. These techniques were largely used for the isolation of new pathogenic viruses that could be cultivated [13]. With progress in the field of molecular biology, PCR-based methods became the main techniques for viral detection from diverse environmental and clinical samples [14]. However, the identification of new or highly divergent viruses that could not be cultivated remained challenging. The development of next-generation sequencing techniques made it possible to sequence all viral genomes in a given sample without previous assumptions about their nature. These techniques, known as viral metagenomics, allowed the discovery of completely new viral species. Currently, the majority of viral metagenomics studies have been performed with DNA viruses [15,16,17]. To our knowledge, the overrepresentation of metagenomic studies performed on DNA viruses compared with RNA viruses is mainly due to technical limitations [18]. In the near future, advances in methodology will certainly enable routine implementation of RNA viral metagenomics studies in humans.
Exploring the Viral Flora in Humans
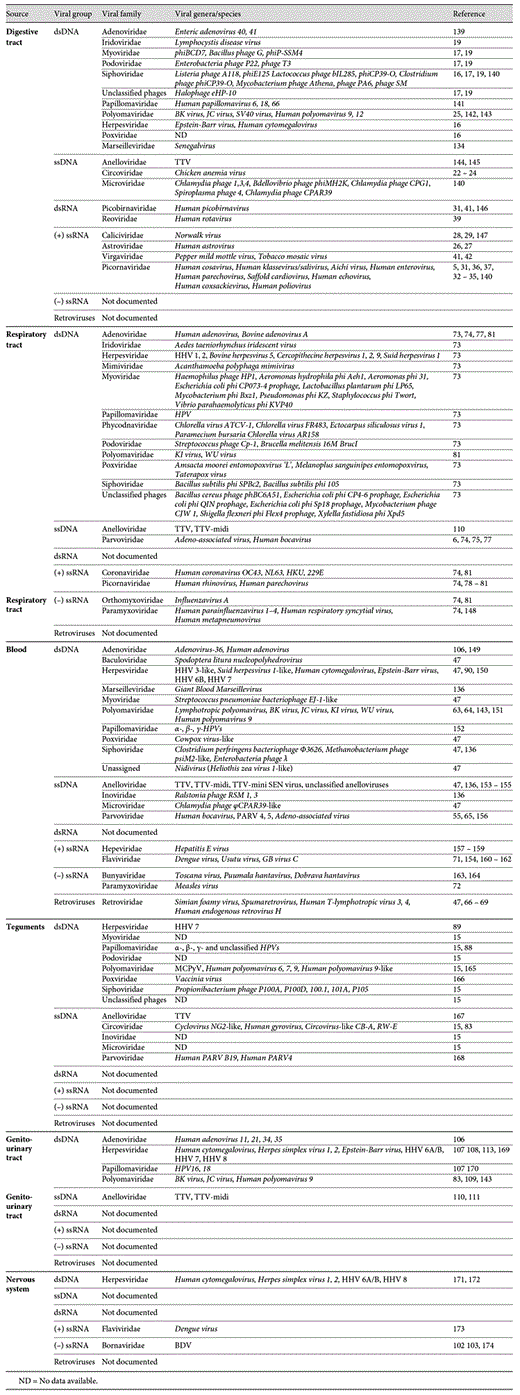
Digestive Tract
The most extensively studied part of the human body with respect to normal viral communities is the human gastrointestinal tract. The study of this system provides several practical advantages; it represents a non-invasive and easy sampling site as well as provides a sufficient amount of material, thereby allowing for the analysis of the viral composition and dynamics in the gut during a normal life. The first large-scale survey of the human gut virome was performed by Rohwer and colleagues [17] 10 years ago. Using partial shotgun sequencing on viral isolates obtained from healthy feces, they detected the presence of bacteriophages that were mainly related to the Siphoviridae family with an estimated 1,200 genotypes. Interestingly, the majority of detected sequences were unclassified, suggesting that the human gut virome was far more complex than expected. The same group undertook a more detailed study of the composition of DNA viruses from the feces of a healthy 1-week-old infant [19]. The results revealed a viral community with extremely low diversity, with an estimated 8 viral genomes corresponding to Podo-, Sipho- and Myo-virus DNA phages. Interestingly, the overall viral community in the human gut proved to be highly dynamic, changing dramatically between 1 and 2 weeks of age. A more detailed analysis of the infant gut was undertaken by Gordon et al. [16], who performed a comparative study of the viruses present in the fecal microbiota of monozygotic twins and their mothers. Interestingly, they found a high prevalence (>75%) of eukaryotic viral genomes in the gut virome, consisting of sequences related to Herpesviridae, Tymoviridae, Reoviridae and Poxviridae. The majority of bacteriophages and prophages were double-stranded DNA (dsDNA) phages and mostly members of the order Caudovirales. Notably, interindividual viral composition was highly divergent between monozygotic twins, whereas the intraindividual viral flora varied little over a year. All studies agreed that phage communities in the human gut played a critical role in the control of the bacterial population. However, deciphering the phage-bacteria-human interactome has only recently begun to emerge. For instance, the viral metagenomics analysis of the oral cavity of healthy individuals performed by Willner et al. [20] showed that phages represent an important reservoir for bacterial virulence genes; thus, phages play a dual role in which they control the bacterial population but also contribute to bacterial pathogenicity and resistance via horizontal gene transfer.
A continually increasing number of eukaryotic single-stranded DNA (ssDNA) viruses in healthy human stool samples has also been identified through high-throughput sequencing or by PCR-based methods [21]. Interesting examples of ssDNA viruses are those from the Circoviridae family. For example, Li et al. [22] found new cycloviruses and circoviruses in human stool samples from Pakistan, Nigeria, Tunisia, and the USA. Another gyrovirus, the Chicken anemia virus, which is an important avian pathogen, was found with a high prevalence (25%) in the feces of Chilean children, suggesting a possible cross-species transmission from farm animals to humans [22,23,24].
Persistent viral shedding of dsDNA viruses of the Polyomaviridae family from the gastrointestinal tract has been reported in several studies. PCR-based detection of the BK, JC and SV40 viruses were identified in healthy children and adults. Viral detection was more frequent in stool samples from children compared with adults. These findings support the hypothesis that the gastrointestinal tract may be a site of Polyomavirus persistence with a possible fecal-oral route of viral transmission [25].
Multiple RNA viruses, generally considered as human pathogens, have also been detected in the normal gut viral flora. PCR-based or metagenomic analyses on ‘healthy' human feces revealed the presence of several eukaryotic viral families, such as Astroviridae [26,27], Caliciviridae [28,29], Picornaviridae, Reoviridae and Picobirnaviridae, as well as plant viral families, such as Virgaviridae. Picornaviridae is the largest (+) ssRNA viral family with more than 12 recognized genera. Viruses belonging to this family have relatively strict host specificity but can infect a wide range of animals, including humans. Cellular tropism ranges from the gut to the central nervous and respiratory systems. In the gut viral flora, Enterovirus (Poliovirus, Echovirus, Coxsackievirus), Kobuvirus (Aichi virus), Parechovirus and Cardiovirus (Saffold virus) [30] have mainly been found, even in a non-pathological context as demonstrated by Kapusinszky [31]. Human Enterovirus type C has also been identified among healthy children [32,33]. Human Cosavirus (for the common stool-associated Picornavirus) and human Salivirus (for the stool Aichi-like virus), which are not yet recognized as new species, have been reported in several studies in stool samples from healthy children [5,34,35,36,37,38]; however, an understanding of their pathogenicity is lacking because they can also be present in cases of gastroenteritis.
Reoviridae and Picobirnaviridae are two dsRNA virus families responsible for gastroenteritis, but both may be present in apparently healthy humans. For example, rotaviruses (Reoviridae, Rotavirus genus) are a major cause of mortality in children under the age of 5 in developing countries, but some genotypes, such as G10P strains, have frequently been associated with asymptomatic neonatal infections in India [39]. The authors reported no significant differences in the sequences obtained from strains infecting symptomatic and asymptomatic neonates, suggesting that host-specific or environmental factors may contribute to the pathogenicity of a virus in a given population. Similar findings concerning Picobirnaviridae were reviewed by Ganesh [40] in 2012. These interesting findings suggest that frequent enteric infections with diverse enteric viruses occur during early childhood and less frequently in adults without clinical symptoms, indicating a change in the virome based on the age and environment of individuals.
Zhang et al. [41] performed the first metagenomic study on the RNA viral community in human feces. They found that the fecal flora was mainly composed of plant-infecting RNA viruses, specifically Pepper mild mottle virus and Tobacco mosaic virus. Plant viruses are generally considered incapable of infecting humans. However, a few studies have reported the presence of plant viral RNA in the human body, including the respiratory system via cigarette use [42] and the gut via contaminated food consumption [43]. Colson et al. [43] noted a higher prevalence of Pepper mild mottle virus in the stools of adults but not children, possibly due to a difference in their diet. In fact, the presence of plant viruses in humans may not represent an infection of the human body but may be due instead to a passive mechanism, such as the ingestion of contaminated food products, suggesting a role of mammals, including humans, as vectors for plant viruses.
The presence of plant viruses in the human gut highlights the fact that the virome may vary between individuals based on diet as demonstrated for bacteria [44]. The virome of the gut may also depend on environmental factors, such as geography, eating habits or ethnic differences, resulting in interindividual variability.
Blood
The human blood and derived products represent a constant need for blood transfusions and medical treatment. However, the blood also represents an important viral reservoir, and some viruses may be pathogenic. Thus, describing the viral flora in the blood has direct consequences for public health. An increasing body of evidence argues that in apparently healthy individuals, the blood is not sterile and may contain many viral species. The majority of the ‘normal' blood viral flora is composed of ssDNA viruses of the Anelloviridae family with Torque teno viruses (TTVs) being the most commonly detected. TTVs are small non-enveloped viruses with icosahedral symmetry that have high genetic diversity. Indeed, the first genus of Anelloviridae, Alphatorquevirus, contains 29 TTV species. Initially detected in a Japanese patient with posttransfusion hepatitis [45], TTVs are now considered commensal with a worldwide distribution [46,47,48]. Although replicative forms of TTV DNA have been detected in peripheral blood mononuclear cells [49], viral loads higher than those in the blood have been identified in the bone marrow, lung, spleen and liver [50]. Thus, it is tempting to speculate that the human blood may play a double role in TTV, both in viral replication and viral dissemination. Several studies have proposed that the main routes for TTV spread are via blood transfusion, oral transmission and sexual contact [48,51,52]. Mother-to-child transmission of TTV has also been reported [53]. These multiple routes of dissemination may contribute to the pandemic nature of TTV infection.
Another frequently detected ssDNA virus family is the Parvoviridae family. Parvoviruses are small non-enveloped viruses with icosahedral symmetry and are approximately 18-26 nm in diameter. Human Parvovirus (PARV)4 was originally detected in the plasma of a person at risk for infection with HIV through intravenous drug use [54]. However, frequent detection of PARV4 and PARV5 in the plasma of apparently healthy blood donors as well as in symptomatic individuals has been reported [55]. In some parts of the world, including sub-Saharan Africa, PARV4 seropositivity is frequently detected with high prevalence in the population [56]. Although infections with PARV4 are not accompanied by long-term viremia, viral DNA sequences can likely be detected in tissues for a long time after exposure [57,58,59], thereby encompassing a form of latency or persistence that is shared with other human PARV, e.g. human PARV B19 and adeno-associated viruses [60,61,62].
Eukaryotic dsDNA viruses have also been detected in blood donors. Egli et al. [63] reported the prevalence of the BK and JC polyomaviruses by testing the blood of 400 donors. Interestingly, they found significant differences between the BK and JC viruses with respect to virus-host interaction and epidemiology. Moreover, lymphotropic Polyomavirus and human Bocavirus (HBoV) have also been frequently found in the peripheral blood of immunocompromised and apparently healthy subjects [64,65].
An increasing number of studies have reported the emergence of new retroviral infections in primate hunters in Africa. Viruses from Retroviridae, such as Simian foamy virus, Spumaretrovirus or Human T-lymphotropic virus 3/4, are naturally acquired by apparently healthy individuals in central Africa after hunting and the butchering of infected meat [66,67]. Moreover, zoonotic retroviruses are frequently detected in the blood of research workers in zoos [68,69,70]. Although the viruses are found in apparently healthy individuals, the long-term consequences of these viral infections must be evaluated. Indeed, it is possible that in the case of persons with immune disorders, these viruses may contribute to the development of chronic pathologies.
RNA viruses are also part of the viral flora in the blood, but they are mainly pathogenic, and in such cases they represent the viremic phase of infection. Only a few examples of circulating ‘asymptomatic' RNA viruses have been reported, but their pathogenicity is not understood. Recently, several arthropod-borne viruses (arboviruses) belonging to the Flaviviridae family, such as Dengue virus, have been detected in the blood of apparently healthy individuals [71]; however, Dengue virus infections can cause undifferentiated fevers and even deaths in some cases. In 2001, Sonoda and Nakayama [72] described circulating Measles virus in peripheral blood mononuclear cells from healthy children exposed to an environment in which measles was circulating. The Measles virus belongs to the Paramyxoviridae family (Morbilivirus genus) and is a major cause of child death in non-vaccinated populations. The authors found a high prevalence of Measles virus (23.4%) in exposed populations, but no detection of viral RNA was observed in unexposed children, suggesting an asymptomatic circulation of the virus.
Respiratory Tract
The respiratory tract is a major gateway of infections for the human body, mainly due to environmental exposure. We distinguish upper respiratory tract infections, which refer to infections of the nasopharynx, larynx, tonsils, sinuses and ears, from lower respiratory tract infections, which refer to infections of the trachea, bronchi and alveoli. The frequency of symptomatic viral respiratory tract infections is higher in young children compared with adults. Although many viruses are responsible for pathologies of the respiratory system (including human rhinoviruses, hRVs, respiratory syncytial virus, influenza and coronaviruses), a number of viruses may be found without any pathological context. In 2009, Willner et al. [73] compared the DNA virome of the upper respiratory tract in people with or without cystic fibrosis to determine whether there was a core respiratory tract virome in non-diseased individuals. In comparison with other viromes, the authors found that the respiratory tract virome had low species richness, most likely due to physical and biological barriers. Although more than 90% of the sequences were unknown, the authors reported the presence of a core set of 19 bacteriophage genomes in the sputum of healthy individuals, reflecting the airborne contamination of each individual. For example, Streptococcus phage Cp-1, Haemophilus influenza phage HP-1 and Brucella melitensis 16 M BrucI prophage were detected along with a random distribution of other phage genotypes. The composition of this phage community may reflect a specific environment, and we can assume that interindividual variability may be due to a difference in environmental exposure. Indeed, some organs, such as the respiratory tract, having frequent contact with the environment, are exposed to different viral communities. In contrast, in cystic fibrosis metagenomes, the pathology appears to favor a phage composition. The study revealed the presence of a core of 20 eukaryotic DNA viral genomes in healthy individuals, mainly composed of adenoviruses, herpesviruses and human papillomaviruses (HPVs). The authors suggested that eukaryotic viral communities in apparently healthy individuals likely represent transient infections that are rapidly cleared by immune cells or viral particles that are removed from the airway via mucociliary clearance.
A metagenomic study conducted in 2012 by Wylie et al. [74] on young children with or without unexplained fever revealed the presence of DNA viruses, including human Parvoviridae viruses (Dependovirus and Bocavirus genera), in the nasal swabs of healthy children. HBoV is the fourth most common virus found in respiratory samples and may be found in healthy subjects [75], but at a lower frequency than it is found in diseases. HBoV may persist in the respiratory tract for a longer period of time than other respiratory agents, resulting in detection of low levels of HBoV [6]. The role of HBoV as a pathogen remains unclear, but the replication mode of this virus, i.e. with the need of ‘helper viruses' (e.g. adenoviruses or herpesviruses), may associate it with respiratory tract diseases [76]. In their metagenomic study, Wylie et al. [74] reported the presence of human adenoviruses in the nasal swabs of healthy children. Adenoviridae (Mastadenovirus genus) viruses are classified into 7 subgroups (A-G) with 55 known serotypes. These viruses usually cause asymptomatic or mild disease in humans, but occasionally some specific subtypes (mainly types 3 and 7) cause severe syndromes, including neurological disorders or deaths in immunocompromised populations or children. In 2011, Heydari et al. [77] reported a case of fatal infection due to the combination of HBoV and human Adenovirus in a previously healthy child. Although a single infection by one of these 2 viruses mainly remains asymptomatic, coinfection with both HBoV and human Adenovirus may result in lethal disease, suggesting that interactions between viruses of the viral communities can lead to pathology.
hRVs are small, non-enveloped, positive ssRNA viruses belonging to the Picornaviridae family (Enterovirus genus). They comprise 3 major genotypes (hRV-A, B and C) that cause a wide range of respiratory illnesses, from mild common colds to serious lower respiratory tract infections [76]. hRVs are also frequently found in asymptomatic children and adults. In 2006, Winther et al. [78] conducted a prospective cohort study of 15 children aged 1-9 years over a 9- to 12-month period. They found a high hRV presence (21%) in the nasal swabs of young children without any reported symptoms. Viral shedding began several days prior to the onset of symptoms and several days after symptoms occurred. They also noted that the maximum duration of viral presence was relatively short (1-3 weeks). Longer hRV presence may be due to reinfection with a new hRV genotype as reported by Van der Zalm et al. [79]. In 2012, Annamalay et al. [80] conducted a similar study on a prospective cohort of 95 children in Australia. No significant difference was observed in the hRV-A prevalence among children with or without symptoms (i.e. a blocked or runny nose).
Wylie et al. [74] revealed the presence of paramyxoviruses (e.g. Paramyxoviridae, Respirovirus and Pneumovirus genera) in the nasal swabs of apparently healthy individuals. They also reported the presence of Influenzavirus A, Parechovirus and Coronavirus in nasopharyngeal swabs, similar to that reported by Van der Bergh et al. [81]. Wylie et al. [74] reported a difference in the abundance of viral sequences with febrile children exhibiting 1.5-fold more viral sequences than samples from afebrile children. They also reported a difference in the diversity of the viral genera present in the samples with a lower diversity found in apparently healthy children. However, no causal relationship between a specific virus and the pathology was found. These observations support the hypothesis that pathology may be due to an imbalance of the microbial communities present in the human body.
Due to the non-invasive nature of the sampling, mainly viromes of the upper respiratory tract of apparently healthy people have been assessed. The viral composition of the lower respiratory tract has been studied using bronchoalveolar lavage samples. One recent study on bronchoalveolar lavage samples from intensive care unit patients identified the presence of viruses from Herpesviridae, Paramyxoviridae and Picornaviridae families [82]. Notably, these viruses were found not only in pneumonia patients, but also in control subjects without pneumonia illness. Thus, additional studies are needed to assess the viral composition of this part of the respiratory system.
Teguments
The human teguments comprise the skin, hair and nails, and play a major role as a barrier protecting the human body from the outside environment. They also represent a complex ecosystem harboring diverse bacterial, fungal and viral species. High-throughput sequencing data on the viral flora of the skin have just begun to be generated. Using Illumina technology, Foulongne et al. [15] detected a high diversity of prokaryotic and eukaryotic viral species in DNA extracts from healthy skin swabs. The most abundant were eukaryotic DNA viruses, such as ssDNA viruses of the Circoviridae family as well as dsDNA viruses of the Polyomaviridae and Papillomaviridae families. Members of Circoviridae (Gyrovirus genus) have been previously reported in the human skin of 4% of healthy persons [83]. Sauvage et al. [83] identified a new virus, the human Gyrovirus, in a skin swab sample from an apparently healthy donor. The host range and infection cycle of human Gyrovirus remains unknown. Other ssDNA viruses from the Parvoviridae family were also found in non-diseased human skin. Although initially reported as the etiological agent of erythema infectiosum, PARV B19 is commonly harbored in apparently healthy human skin. Bonvicini et al. [84] found the prevalence of B19 to be 25% in apparently healthy skin biopsies. Interestingly, the group found that young subjects had a significantly higher rate of B19 viremia compared with adults, suggesting that long-term viral persistence may be the common outcome after primary infection.
Polyomaviruses are also common skin viruses. They have a circular dsDNA genome of approximately 5,000 bp that is surrounded by a non-enveloped icosahedral capsid. Polyomaviruses were first described in 1953 in mice, but since then these viruses have been detected in other vertebrate species, including humans. In humans, a new Polyomavirus, Merkel cell Polyomavirus (MCPyV), was recently identified [85,86]. The presence of MCPyV in human skin has been associated with an aggressive form of skin cancer, Merkel cell carcinoma (MCC). MCPyV infections are found in 80% of MCCs. However, MCPyV and two newly identified polyomaviruses, HPyV6 and HPyV7, are also frequently shed from apparently healthy human skin [15,87]. In the case of MCC, the accumulation of deleterious mutations in the MCPyV genome, including the viral T antigen gene, render the virus non-infectious. Thus, the oncogenic role of MCPyV does not necessary reflect its lifestyle but rather the consequence of deleterious viral mutations. Other dsDNA viruses that are associated with neoplastic development have also been identified in healthy skin. Detection of α- and β-HPVs as well as human Herpesvirus (HHV)7 has been reported recently in skin biopsies [88,89]. HHV7 was initially isolated from CD4+ T cells obtained from peripheral blood lymphocytes of an apparently healthy individual [90] and was later associated with primary cutaneous T cell lymphomas (CTCLs). However, the low prevalence of HHV7 in CTCL as well its presence in healthy skin biopsies suggests that HHV7 may not be the primary cause of CTCL [89,91].
Bacteria-infecting viruses are also frequently found in the human skin and most likely play an important role in controlling the bacterial population. Using viral metagenomics, viruses belonging to the Myoviridae, Siphoviridae, Microviridae, Podoviridae and Inoviridae families were identified, and viruses from the Siphoviridae and Microviridae families were the most abundant. One common phage genera present in healthy human skin consisted of bacteriophages infecting Propionibacterium acnes(Siphoviridae family). The P. acnes bacterium represents a dominant member of the skin microflora and has also been implicated in the pathogenesis of acne. Multiple P. acnes bacteriophages isolated from the sebaceous follicles of healthy skin donors have recently been characterized [9]. Interestingly, these phages showed reduced genetic variability with a broad range of infecting bacterial strains, suggesting the existence of evolutionary constraints that preserve the homogeneity of the phage population.
Nervous System
Little information is available concerning the viral flora in the human nervous (central and peripheral) system in apparently healthy conditions. Examples of neurotropic human viruses are the Herpes simplex virus (HSV)1 and HSV2, which belong to the Herpesviridae family. These viruses have a dsDNA genome located within an icosapentahedral capsid surrounded by an amorphous protein-like material (known as the tegument), which is in turn encapsulated by an envelope consisting of polyamines, lipids and glycoproteins [76]. Genetically, HSV1 and HSV2 are closely related, sharing approximately 70% homology. During primary infection, the virus enters the nerve endings at the peripheral mucocutaneous region. The viral capsid is brought via fast axonal transport into the neuronal cell body of the dorsal root ganglia or the trigeminal ganglia. The viral DNA enters the nucleus of the neuron where it enters a latent state [92]. Notably during this period, two latency-associated transcripts are expressed [93]. Latency-associated transcripts have been shown to have antiapoptotic activity, thereby sustaining the survival of neurons. This activity illustrates the virus-to-host adaption and the benefit of a latent persistence in the nervous system. Although HSV1 and HSV2 are associated with clinical complications, the majority of the infections remain asymptomatic for years or even decades. Indeed, under immunocompetent conditions, the reactivated infection usually remains confined to the vicinity of a single dorsal root ganglion. It has been estimated that asymptomatic reactivation of HSV1 may exceed clinical recrudescence, and asymptomatic HSV2 shedding can occur in more than two-thirds of seropositive individuals [94,95].
Another interesting example of a neurotropic virus is the Borna disease virus (BDV), which is part of the Bornaviridae family. BDV is an 80- to 100-nm enveloped virion, containing an 8.9-kb (-) ssRNA genome that replicates in the cell nucleus [96,97]. In vitro BDV induces non-cytopathic chronic infections in neurons [98]. BDV infection was first identified in horses, and natural infections with BDV were subsequently detected in other vertebrates, including humans [99]. In this context, BDV was suggested as a causative agent of diverse human psychiatric disorders [100,101,102]. Despite these findings, the seroprevalence of the virus in healthy control groups makes the causal relationship between BDV infection and brain disorders hardly verifiable [103]. Recently, endogenous BDV sequences homologous to the viral nucleoprotein were detected in several mammalian species, including humans, suggesting an ancient cohabitation with a BDV ancestor [104,105]. Overall, further efforts, especially using a viral metagenomics approach, should be put into the study of the viral diversity of the human nervous system.
Genito-Urinary Tract
The viral flora of the genito-urinary tract has been mainly studied in pathological situations, and gaps in the knowledge of the viral flora in apparently healthy conditions need to be filled. Asymptomatic shedding from the genito-urinary tract was reported mainly for dsDNA eukaryotic viruses of the Adenoviridae, Herpesviridae, Papillomaviridae and Polyomaviridae families with the exception of ssDNA viruses of the Anelloviridae family [83,106,107,108,109,110,111]. In the case of polyomaviruses, it appears that viral excretion was correlated with the host immune status. Indeed, Csoma et al. [112] detected KI virus and WU virus in the urine of renal transplants but not in the control groups. Moreover, immunosuppression due to pregnancy led to a higher prevalence of BK virus in urine samples in pregnant women compared to non-pregnant women [113].
Multiple herpesviruses were also frequently detected in the genito-urinary tract, especially in the semen of apparently healthy donors. In this case it appears that some herpesviruses, such as human Herpesvirus 6 A/B or the Cytomegalovirus, were able to attach to the sperm head with an intact acrosome [108,113]. Thus, given the potential risk some herpesviruses may represent to the newborns, additional research is required to evaluate the impact of this asymptomatic shedding from herpesvirus-positive donor semen.
Broad Distribution and Impact of Papillomaviruses in the Human Body
When examining the repartition of viruses according to their distribution in the human body (fig. 1), one can note that DNA viruses of Herpesviridae, Papillomaviridae, Polyomaviridae and Anelloviridae families are present both in the respiratory tract, the gut, the skin, the blood and the genito-urinary tract. One hypothesis may be related to the viral-host adaptation process. For sustained infection, viruses need to have wide range of body repartition allowing them to proliferate efficiently.
Description of the viral composition in the human body. Table summarizing the viral families documented (in green) or not documented (in violet) in each human system.
Description of the viral composition in the human body. Table summarizing the viral families documented (in green) or not documented (in violet) in each human system.
Papillomaviruses represent good examples of pleiotropic human viruses in the human body. Papillomaviruses are 55- to 60-nm non-enveloped DNA viruses composed of a single, circular dsDNA molecule. This viral family consists of more than 120 different HPV types, about 40 of which are sexually transmitted HPVs and a dozen have been identified as the causative agents of cervical, anal, vaginal and penile cancer [114]. HPVs are present in more than 99% of cervical cancers, and HPV type 16 (HPV-16) and HPV-18 are the predominant causes of infection in these cases [115]. These two HPV types are indeed associated with 70% of all cervical cancers with predominance of HPV-16 accounting for about 50% of cases [116]. More recently, papillomaviruses were linked to head and neck malignancies as well. In these cases, the primary causes for these carcinomas were attributed to alcohol and tobacco consumption. However, the number of respiratory and digestive tract cancers caused by HPV infections is constantly increasing [117,118,119]. Indeed patients with HPV-positive carcinoma are generally younger adults and not alcohol and tobacco users. These carcinomas are mainly localized in the oropharynx and in particular at the tonsils. HPV is found with a prevalence of 40-90% of the oropharynx cancers, depending on the geographical distribution [120,121,122].
HPVs have cellular tropism for the stratified squamous epithelia. Although the exact mechanism of Papillomavirus tumorigenesis is not fully elucidated it is generally accepted that this effect is mediated through E6, E7 viral proteins which control cell death and proliferation [123,124,125]. Despite the oncogenic properties of these viruses, the majority of HPV infections remain asymptomatic, and they are cleared by most people without medical consequences. Indeed, the clearance of HPV 18 months postinfection in the male population is 100%, whereas in females it is 97%, suggesting that in the case of an immunocompetent host, HPV infection manifests as a transient phenomenon [126,127]. The significance of their presence in an apparently healthy context remains unknown.
The Human Megavirome
dsDNA viruses with large genomes (also known as giant viruses) represent a monophyletic group of viruses classified under the order of Megavirales [128]. Giant viruses are divided into seven viral families, including Poxviridae, Iridoviridae, Ascoviridae, Mimiviridae, Phycodnaviridae, Asfaviridae and the recently described Marseilleviridae [128,129]. These viruses infect a wide range of eukaryotes, including phagocytic protists and humans [130]. In humans, members of only two of the families, Poxviridae and Mimiviridae, have been linked to disease [131,132,133]. With next-generation sequencing technologies, an accumulating body of evidence indicates the presence of these viruses in non-pathological conditions. For instance, a metagenomics study carried out by Willner et al. [73] detected multiple DNA sequences related to Poxviridae, Iridoviridae, Mimiviridae and Phycodnaviridae. Moreover, several studies have identified the presence of giant viruses in the human gut in both adults and babies [16,19,134]. Breitbart et al. [19] detected sequences homologous to Lymphocystis disease virus (Iridoviridae), a fish-infecting pathogen, whereas Gordon et al. [16] detected previously uncharacterized Pox-related viral sequences in the infant gut.
Recently, a new giant virus closely related to Marseilleviridae, Senegalvirus, was recovered from a stool sample of a 20-year-old Senegal man [134]. Senegalvirus was detected by ultradeep sequencing and was isolated using an amoebal coculture. The Senegalvirus dsDNA genome is approximately 373 kbp in length, making this genome the largest among marseilleviruses. In the same stool, sequences related to the giant Mimivirus were also found [135].
Another virus closely related to the Marseilleviridae family was recently identified in human blood. This new virus, Giant Blood Marseillevirus (GBM), has an estimated 357-kbp dsDNA genome surrounded by a 200-nm capsid (fig. 2). The GBM virus was initially isolated from a blood transfusion pocket using a 0.45-µm filter coupled with high-throughput sequencing from a 32-year-old healthy female donor [136]. Further testing identified concomitant elevated IgG levels and viral DNA in some blood donors, suggesting the persistence of the GBM virus in the blood. Interestingly, GBM was found to infect and replicate in human T cells, but not in amoebas.
Detection of GBM. a Negative staining of a Marseillevirus-like particle (arrow) present in the virus-purified fraction of serum from blood donor No. 27725. b Epifluorescent microscopy images from fluorescent in situ hybridization of GBM in serum from blood donor No. 27725. The DNA probe was synthesized using the Marseillevirus genomic region, orf 152-153, and is marked in green; nuclear staining with DAPI dye is in blue. Scale bar = 10 µm.
Detection of GBM. a Negative staining of a Marseillevirus-like particle (arrow) present in the virus-purified fraction of serum from blood donor No. 27725. b Epifluorescent microscopy images from fluorescent in situ hybridization of GBM in serum from blood donor No. 27725. The DNA probe was synthesized using the Marseillevirus genomic region, orf 152-153, and is marked in green; nuclear staining with DAPI dye is in blue. Scale bar = 10 µm.
In the environment, the majority of Marseillevirus-related viruses have been isolated from aquatic and soil environments, suggesting the possibility of a common infectious route in humans [129,137,138]. Although they are found in non-pathological conditions, the consequences of long-term viral persistence should be further evaluated.
Conclusion
The Human-Virus Interactome Goes Beyond Simple Parasitism
Viruses and humans coexist and are constantly interacting. Historically, viruses have been classified as strict intracellular pathogens. However, with the development of new technologies for viral detection, it has become clear that their presence within the healthy human body goes beyond simple parasitism (fig. 1, 3; table 1). The role of the majority of eukaryotic viruses in the healthy human body remains unclear. Although the long-term consequences of viral presence in terms of pathological conditions should be evaluated, it is possible that such viruses may be considered commensals. In other cases, it is not a single virus that is pathogenic for humans but the coinfection with different viruses. The combination of HBoV and Adenovirus represents a good example of such coinfection [77]. The presence of viruses in the human body without any pathological context could also be beneficial for the body or for the human microbial flora. An example of symbiosis between viruses and the human host is the phage communities of the human gut, and these communities may play an important role in the control of the bacterial population. Conversely, a negative interaction (negative for humans) is that phages may represent an important reservoir for bacterial resistance genes and may contribute to bacterial pathogenicity via horizontal gene transfer [20]. As a result, the boundaries between mutualistic and pathogenic viruses remain elusive and are most likely highly dynamic throughout life [2].
The human virome in non-pathogenic conditions: distribution of the viral families found in the major human systems. Each viral group is represented with a unique color.
The human virome in non-pathogenic conditions: distribution of the viral families found in the major human systems. Each viral group is represented with a unique color.
The human-virus interactome should be considered as a complex web of interactions, defined by multiple factors. These factors can be classified into three categories: virus-specific (e.g. viral genotype, replication mode, host range, abundance), host-specific (e.g. genetic background, age, immune system) and environment-specific (e.g. geographic location, demographic distribution, animal proximity). In the case of the human virome under healthy conditions, the weight of each factor lays at a metastable equilibrium point, allowing viruses and humans to coexist naturally (fig. 4). A change in one of these parameters could lead to the development of disease conditions or the clearance of the virus from the body. From a medical point of view, a new paradigm is thus emerging; if we define an illness as a disruption of the normal ‘healthy' virome, then the restoration of this equilibrium should be the goal of medical treatment, not the elimination of all non-human microorganisms.
Human virus metastable equilibrium in non-pathogenic conditions. Schematic representation of the steady state of the human virome in non-pathogenic conditions as regulated by three major factors (virus, host and environment). The disequilibrium of this metastable system leads either to viral spreading or to viral clearance.
Human virus metastable equilibrium in non-pathogenic conditions. Schematic representation of the steady state of the human virome in non-pathogenic conditions as regulated by three major factors (virus, host and environment). The disequilibrium of this metastable system leads either to viral spreading or to viral clearance.
Acknowledgements
This work was supported by the Starting Grant No. 242729 from the European Research Council awarded to C. Desnues.
Disclosure Statement
The authors declare that they have no conflict of interest.
References
N.P. and S.T. contributed equally to this work.