Abstract
Previous comparative research has attributed interspecific variation in eye size among mammals to selection related to visual acuity. Mammalian species have also been hypothesized to differ in visual acuity partly as a result of differences in ecology. While a number of prior studies have explored ecological and phylogenetic effects on eye shape, a broad comparative analysis of the relationships between visual acuity, eye size and ecology in mammals is currently lacking. Here we use phylogenetic comparative methods to explore these relationships in a taxonomically and ecologically diverse sample of 91 mammal species. These data confirm that axial eye length and visual acuity are significantly positively correlated in mammals. This relationship conforms to expectations based on theoretical optics and prior analyses of smaller comparative samples. Our data also demonstrate that higher visual acuity in mammals is associated with: (1) diurnality and (2) predatory habits once the effects of eye size and phylogeny have been statistically controlled. These results suggest that interspecific variation in mammalian visual acuity is the result of a complex interplay between phylogenetic history, visual anatomy and ecology.
Introduction
Mammals exhibit substantial variation in visual acuity (i.e. the ability to resolve spatial details), ranging from the low acuity vision of microchiropteran bats and small rodents (0.4-1.0 cycles per degree, or cpd) to the highly acute vision (30-64 cpd) of diurnal anthropoid primates [Walls, 1942; Pettigrew et al., 1988; Kirk and Kay, 2004]. The evolution of higher visual acuity in certain clades (e.g. primates) is hypothesized to reflect an increased reliance on vision in meeting basic needs, such as finding food and avoiding predators [Walls, 1942; Hughes, 1977; Kay and Kirk, 2000; Ross, 2000; Kirk and Kay, 2004; Veilleux and Kirk, 2009; Hall et al., 2012]. At the same time, the lower acuity of some other mammalian clades (e.g. microchiropteran bats, rodents) may reflect an increased dependence on nonvisual senses [Walls, 1942; Hutcheon et al., 2002; Diamond et al., 2008; Hayden et al., 2010]. Thus, identifying the factors that influence visual acuity is critical for understanding mammalian sensory ecology.
In mammalian eyes, theoretical optics predicts that absolute eye size will influence a number of different aspects of visual functionality [Walls, 1942; Hughes, 1977]. In particular, eye axial diameter (‘eye length') plays a key role in determining retinal image size [Hughes, 1977]. If eye shape is held constant, an increase in eye length produces a longer posterior nodal distance (PND) and increases the size of the retinal image (fig. 1) [Hughes, 1977; Pettigrew et al., 1988; Ross, 2000]. Larger retinal images will generally be sampled by a greater number of independent sampling units (i.e. ganglion cell receptive fields), such that eye length should be positively correlated with visual acuity [Walls, 1942; Kirschfeld, 1976; Hughes, 1977; Heesy and Hall, 2010]. As a result, studies that calculate visual acuity based on PND and peak ganglion cell density typically use measurements of eye length to estimate PND [Hughes, 1977; Pettigrew et al., 1988; Arrese et al., 1999; Pettigrew et al., 2010]. Species with absolutely longer eyes are therefore expected to have higher acuity than species with shorter eyes. Some support for this prediction has been provided by comparative analyses of visual acuity and eye length in mixed samples of mammals and birds [Kiltie, 2000; Heesy and Hall, 2010]. However, these studies employed relatively small mammalian samples with limited taxonomic and ecological diversity. For example, Kiltie [2000] demonstrated a positive correlation between acuity and eye length in a sample of 14 nocturnal mammals and 3 diurnal anthropoids. A similar result was obtained by Heesy and Hall [2010] using a sample of 14 nocturnal and 11 diurnal mammal species, but the relationship between acuity and eye length was less clearly linear than that shown by Kiltie [2000].
Effect of eye size on retinal image size. In this schematic example, two eyes have identical shape but differ in axial diameter. Here the larger eye has an axial diameter approximately twice that of the smaller eye. Both eyes are positioned so that a visual target (double-headed arrow) of length Y is the same distance from the posterior nodal point (black circle; position approximate). In this example, the retinal image of the target in the large eye (2X) is twice the length of the retinal image in the smaller eye (X).
Effect of eye size on retinal image size. In this schematic example, two eyes have identical shape but differ in axial diameter. Here the larger eye has an axial diameter approximately twice that of the smaller eye. Both eyes are positioned so that a visual target (double-headed arrow) of length Y is the same distance from the posterior nodal point (black circle; position approximate). In this example, the retinal image of the target in the large eye (2X) is twice the length of the retinal image in the smaller eye (X).
Comparative studies of eye length and other aspects of visual morphology have linked increased visual acuity to a variety of ecological factors, including diel activity pattern, diet and speed of locomotion [Walls, 1942; Hughes, 1977; Pettigrew et al., 1988; Arrese et al., 1999; Kirk and Kay, 2004; Peichl, 2005; Heard-Booth and Kirk, 2012]. Diel activity pattern (i.e. the time of day that a species is habitually active) in particular is predicted to exhibit a strong relationship with acuity because adaptations that enhance visual sensitivity in low light are generally incompatible with high acuity [Walls, 1942; Hughes, 1977; Land and Nilsson, 2012]. Consequently, diurnal mammals are expected to have higher acuity than cathemeral or nocturnal species [Walls, 1942; Hughes, 1977; Pettigrew et al., 1988; Heesy and Hall, 2010]. Comparative studies have provided some support for this prediction in marsupials [Arrese et al., 1999; Arrese et al., 2000; Arrese et al., 2002] and strepsirrhine primates [Veilleux and Kirk, 2009]. Kiltie [2000] also reported that diel activity pattern has a strong influence on the relationship between acuity and eye length, but the sample of diurnal mammals in this analysis consisted entirely of anthropoid primates. Diurnal anthropoids have highly derived visual anatomy and substantially higher visual acuity than other diurnal mammals [Ross, 2000; Kirk, 2004; Kirk and Kay, 2004; Ross and Kirk, 2007; Hall et al., 2012], suggesting that the difference in visual acuity between diurnal and nocturnal mammals observed by Kiltie [2010] may be attributable to factors other than diel activity pattern per se. Indeed, the data presented by Heesy and Hall [2010] appear to show little difference between diurnal and nocturnal nonanthropoid mammals in the relationship between eye length and acuity. Accordingly, the relationship between diel activity pattern and visual acuity across mammals remains poorly understood.
Other ecological factors have also been suggested to influence variation in mammalian visual acuity. For example, mammals that engage in visually mediated hunting are predicted to have higher acuity than more herbivorous species [Arrese et al., 1999, 2000; Ross, 2000; Tetreault et al., 2004; Kirk, 2006a; Ross and Kirk, 2007; Heesy, 2008; Veilleux and Kirk, 2009]. The observation that maximum running speed (MRS) and eye length are strongly positively correlated in mammals has also led to the suggestion that speed of locomotion influences visual acuity [Hughes, 1977; Heard-Booth and Kirk, 2012]. However, these hypotheses regarding the effect of diet and locomotion on visual acuity have not been tested in a broad comparative analysis.
While prior comparative research has documented phylogenetic and ecological influences on mammalian eye size [Ross and Kirk, 2007; Heard-Booth and Kirk, 2012], these studies have generally assumed that the evolution of larger eyes is functionally associated with selection for higher visual acuity. Here we test this expectation by examining the relationship between eye length and visual acuity in a taxonomically and ecologically diverse sample of 91 species from 14 mammalian orders. After statistically controlling for phylogeny and interspecific differences in eye size, we next evaluate the relationships between visual acuity and diel activity pattern, diet and MRS. In so doing, we have two primary objectives. First, we seek to precisely quantify the effect of eye size on visual acuity in mammals using a large comparative dataset that is less constrained by sampling biases than prior analyses. Second, we seek to determine whether diurnality, active predation and fast running speeds are associated with higher visual acuity even after accounting for the effects of eye size on acuity.
Materials and Methods
Comparative Dataset
We compiled published data on maximum visual acuity, eye length and body mass for 91 mammal species (online suppl. table 1; see www.karger.com/doi/10.1159/000357830 for all online suppl. material). We included both behaviorally measured visual acuity and estimates of visual acuity derived from retinal anatomy and eye size. When anatomical and behavioral acuity were both available for a species, we used behavioral measurements because they better represent visual function [Arrese et al., 2000]. Anatomical estimates of acuity represent a theoretical maximum and tend to slightly overestimate acuity measured behaviorally [Pettigrew et al., 1988; Arrese et al., 1999].
For 14 species (Bos taurus, Capra hircus, Dama dama, Sus scrofa, Acinonyx jubatus, Canis lupus, Sarcophilus harrisi, Didelphis virginiana, Macropus fuliginosus, Setonix brachyurus, Alouatta caraya, Chlorocebus aethiops, Saguinus midasand Choloepus didactylus), we modified established protocols for calculating anatomical acuity based on published eye lengths and either peak retinal ganglion cell density or peak cone density [Pettigrew et al., 1988; Arrese et al., 1999]. Following Pettigrew et al. [1988], we first estimated PND from eye length using a multiplicand k, which differs according to diel activity pattern. Different researchers have proposed varying values for k. Hughes [1977] initially utilized k = 0.60 for all taxa, which Pettigrew et al. [1988] subsequently revised to account for differences in eye shape with activity pattern (k = 0.52, 0.57 and 0.67 for nocturnal, cathemeral and diurnal species, respectively) based on a comparative sample of 14 vertebrate species. This method for estimating PND accounts for the fact that eye morphology in mammals varies predictably with diel activity pattern [Kirk, 2004; Hall et al., 2012]. More recently, Schmitz [2009] used an expanded dataset of 23 species to revise these values of k to 0.55, 0.63 and 0.66 for nocturnal, cathemeral and diurnal species, respectively. However, this source incorrectly classified ostriches (Struthio camelus)as cathemeral rather than diurnal [Williams, 1993] and European rabbits (Oryctolagus cuniculus) as nocturnal rather than cathemeral [Lombardi et al., 2003]. We therefore reclassified these two species, yielding a total comparative sample of 6 nocturnal, 6 cathemeral and 11 diurnal vertebrate species for which both PND and eye length can be used to calculate k [cf. table 1 in Schmitz, 2009]. Our revised values for k based on this sample are, thus, 0.547 for nocturnal species, 0.623 for cathemeral species and 0.643 for diurnal species. After estimating PND based on eye length, we calculated the retinal magnification factor (RMF) as (2π·PND)/360. In most mammals, multiple cone photoreceptors may pool their inputs to each ganglion cell (i.e. retinal summation) even in the central retina. As a result, the density of ganglion cells represents a limiting factor for acuity [Pettigrew et al., 1988; Kay and Kirk, 2000]. By contrast, diurnal haplorhines exhibit no retinal summation in the fovea [Kirk and Kay, 2004], so acuity is limited by the density of cones [Pettigrew et al., 1988]. Accordingly, we calculated visual acuity for diurnal haplorhines using the formula (RMF·√peak cone cell density)/2, and for all other taxa using the formula (RMF·√peak ganglion cell density)/2 [Pettigrew et al., 1988].
Where available, we compiled data on diel activity pattern, MRS and diet for each species (online suppl. table 1). Diel activity pattern was categorized as diurnal, cathemeral or nocturnal [Ross and Kirk, 2007; Hall et al., 2012]. We used the highest MRS reported for available species. We restricted our dietary analysis to comparisons of herbivorous species (‘herbivore') and species described in the literature as actively catching moving prey (‘active predator'), following categories similar to Garamszegi et al. [2002]. We excluded species for which hunting style was unavailable.
Analyses of Visual Acuity and Eye Length
We used phylogenetic generalized least-squares (PGLS) regression to investigate the relationship between eye length and visual acuity [Garland and Ives, 2000]. PGLS analyses were performed in R v.2.15.2 [R Development Core Team, 2011] using the geiger and caper packages [Orme et al., 2010; Harmon et al., 2008]. Tree topology and branch lengths follow Bininda-Emonds et al. [2007]. Because eye length is involved in calculations of anatomical acuity [albeit transformed by the factor k according to diel activity pattern; Pettigrew et al., 1988], there is some concern of circularity in using anatomically derived estimates to explore the relationships between acuity and eye length. Previously, Kiltie [2000, p. 229] found that results ‘did not differ significantly' when using anatomical or behavioral estimates to explore the relationship between eye length and acuity. To compare our behavioral and anatomical datasets, we calculated separate PGLS regressions of: (1) behavioral acuity on eye length, (2) anatomical acuity on eye length and (3) the combined dataset of behavioral and anatomical acuity on eye length. For all PGLS regressions, we calculated Pagel's lambda [Freckleton et al., 2002] in caper to estimate the influence of phylogenetic relationships on our data.
Because haplorhine primates exhibit specializations for high visual acuity that are unique among mammals [e.g., a retinal fovea; Kirk and Kay, 2004], we expect haplorhines to differ from other mammals in the relationship between eye length and acuity [Kiltie, 2000; Heesy and Hall, 2010]. Accordingly, we compared the residuals of haplorhine primates, strepsirrhine primates, and nonprimate mammals from the combined PGLS regression line of acuity on eye length. Differences in the residuals of these three groups were assessed using a Kruskal-Wallis test followed by one-tailed post hoc Wilcoxon tests.
Analyses of Visual Acuity and Ecology
We next examined the effects of diel activity pattern, diet and MRS on the relationship between acuity and eye length. Specifically, we tested whether these ecological factors influence variation in acuity when phylogeny and eye length have been statistically controlled. We restricted these ecological analyses to the behavioral acuity dataset. Several groups of taxa that were included in our examination of eye length and acuity were excluded from our ecological analyses. First, semiaquatic and fossorial species were excluded from all ecological analyses because they occupy visual environments that are fundamentally different from those encountered by other mammalian species in our sample. Second, we excluded haplorhine primates from all ecological analyses because they exhibit a suite of derived adaptations for very high visual acuity that are not found in other mammals [Ross, 2000; Kirk, 2004; Kirk and Kay, 2004; Ross and Kirk, 2007; Hall et al., 2012]. Third, we excluded microchiropteran bats from our analyses of diet because they have evolved alternate sensory adaptations (active echolocation) for hunting moving prey [Schnitzler and Kalko, 2001]. With these exclusions, we have attempted to restrict our ecological comparisons to groups of species that do not differ fundamentally in: (1) visual bauplan or (2) visual ecology. Furthermore, it is important to note that some faunivorous and omnivorous mammals may rely more on nonvisual senses during foraging [Walls, 1942; Hutcheon et al., 2002; Diamond et al., 2008; Hayden et al., 2010]. Accordingly, we limited our dietary analyses to compare herbivores with predators that actively catch moving prey. In the latter group, vision is expected to play an important role in compensating for the evasive movements of prey.
We employed two approaches to explore ecological effects on acuity: (1) traditional nonparametric tests and (2) PGLS multivariate modeling. For the nonparametric tests, we calculated residual acuity for each species using PGLS regressions of acuity on eye length for the behavioral dataset. Residual acuity is expected to be mainly a function of differences in retinal anatomy because it represents the portion of interspecific variation in visual acuity that is unexplained by eye length. We then compared variation in residual acuity between ecological categories. We also explored how raw visual acuity (uncorrected for eye length or phylogeny) varies with ecology. Following predictions from visual anatomy, we tested for variation in residual and raw acuity with diel activity pattern (diurnal, cathemeral, nocturnal) using a Kruskal-Wallis test followed by one-tailed post hoc Wilcoxon tests. We similarly compared residual and raw acuity across the two dietary groups (herbivores, active predators) using one-tailed Wilcoxon tests. We used χ2 tests to confirm that the proportion of species in each diel activity pattern did not significantly differ between diet categories. We examined the relationships between MRS and both raw acuity and residual acuity using Spearman rank correlations.
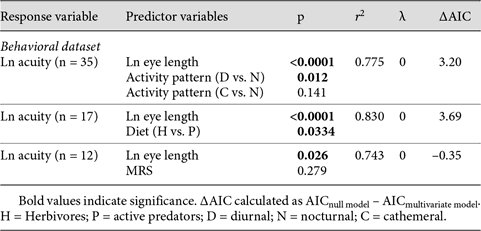
We also calculated three separate PGLS multivariate models with visual acuity as the response variable and with eye length and one of three ecological variables (diel activity pattern, diet or MRS) as co-predictor variables. For each of these PGLS multivariate models with an ecological variable as a co-predictor, we also calculated a null PGLS bivariate regression of acuity and eye length using the same subset of species. We compared the fit of the multivariate models and null models using Akaike Information Criterion (AIC) values and ANOVA. When ΔAIC was <2, the models were considered equivalent [Burnham et al., 2011; Symonds and Moussalli, 2011]. Multivariate models with ΔAIC of 3-7 were considered possibly better than the null, while multivariate models with ΔAIC >10 were considered substantially better than the null model [Burnham et al., 2011; Symonds and Moussalli, 2011]. We also compared the null and multivariate models using the sequential sum of squares with the anova.pgls function in caper.
Results
Visual Acuity and Eye Length
Our comparative sample documents substantial variation in visual acuity both within and across mammalian taxonomic groups (fig. 2). In general, microchiropteran bats exhibit the lowest visual acuity in our sample (median 0.6 cpd, range 0.1-1.9 cpd), while haplorhine primates have the highest visual acuity (median 46 cpd, range 8.3-64.3 cpd). Visual acuity for the majority (∼91%) of nonhaplorhine taxa ranged between 0.1 and 10.4 cpd (± 1 standard deviation). We also identified several outlier species that have substantially higher visual acuity than the other species in their taxonomic groups (fig. 2), including the giraffe (Giraffa camelopardalis; 25.5 cpd), cheetah (Acinonyx jubatus; 23 cpd) and western grey kangaroo (Macropus fuliginosus; 11.2 cpd).
The PGLS regression of behavioral acuity on eye length in 42 species reveals a significant positive relationship between the two variables (fig. 3a; table 1). Thus, as eye size increases, visual acuity also tends to increase. According to this regression, ∼35% of the variation in visual acuity between mammalian species is attributable to variation in eye length (table 1). Seven small-eyed species plot well below the behavioral regression line (fig. 3a), suggesting that they have lower acuity than expected for their eye length. These taxa include 4 micochiropteran bats (Myotis mystacinus, M. daubentonii, Artibeus jamaicensis, Phyllostomus hastatus) and 3 nocturnal or fossorial rodents (Mesocricetus auratus, Mus musculus, Peromyscus maniculatus). Diurnal haplorhine primates also plot in a cluster above the regression lines, indicating that they have high acuity for their eye length. Nonetheless, the taxa in these low-acuity and high-acuity groups were not identified as outliers in the model [defined as having a studentized residual ±3; Jones and Purvis, 1997]. A separate PGLS regression of anatomical acuity on eye length in 49 species indicates a similar significant positive relationship between the two variables (table 1). The slopes of the behaviorally and anatomically derived regression lines have overlapping 95% confidence intervals (behavioral 0.58-1.36; anatomical 0.85-1.32), suggesting that there is a consistent relationship between acuity and eye length regardless of how acuity is measured (table 1). Moreover, a PGLS multivariate model of acuity and eye length with measurement type (behavioral, anatomical) as a co-predictor variable found that measurement type is not a significant factor in the model (p = 0.072). When all visual acuity data are combined, the PGLS regression of acuity on eye length is similar to regressions obtained using the behavioral and anatomical subsets (table 1) and falls entirely within their 95% confidence intervals.
Effects of eye length and phylogeny on visual acuity in 91 mammals. a PGLS regressions of acuity and eye length for behavioral, anatomical and combined datasets. b Quartile boxplots of residual acuity calculated for haplorhine primates, strepsirrhine primates and nonprimate mammals using the combined acuity regression. Whiskers represent highest and lowest values that are not outliers (closed circles; defined as >1.5 times the interquartile range). Haplorhine outliers: owl monkeys (Aotus azarae, A. trivirgatus) and tarsier (Tarsius syrichta). Nonprimate mammal outliers: fruit bat (A. jamaicensis) and greater spear-nosed bat (P. hastatus).
Effects of eye length and phylogeny on visual acuity in 91 mammals. a PGLS regressions of acuity and eye length for behavioral, anatomical and combined datasets. b Quartile boxplots of residual acuity calculated for haplorhine primates, strepsirrhine primates and nonprimate mammals using the combined acuity regression. Whiskers represent highest and lowest values that are not outliers (closed circles; defined as >1.5 times the interquartile range). Haplorhine outliers: owl monkeys (Aotus azarae, A. trivirgatus) and tarsier (Tarsius syrichta). Nonprimate mammal outliers: fruit bat (A. jamaicensis) and greater spear-nosed bat (P. hastatus).
Our estimates of Pagel's lambda suggest that there is a strong phylogenetic influence on the relationship between eye size and visual acuity (table 1). Lambda is equal to 1.00 in the PGLS regression of behavioral acuity on eye length, indicating that the distribution of character states among taxa is highly correlated with our chosen phylogeny. However, lambda is significantly less than 1 for both the anatomical (λ = 0.75, p = 0.007) and combined datasets (λ = 0.71, p < 0.0001). The influence of phylogeny is particularly evident for haplorhine primates. Controlling for eye size, residual acuity differs significantly between haplorhine primates, strepsirrhine primates and nonprimate mammals (Kruskal-Wallis: χ2(2) = 30.90, p < 0.0001). In particular, haplorhines have significantly higher residual acuity compared to both nonprimate mammals (Wilcoxon: W = 958, p < 0.0001) and strepsirrhines (Wilcoxon: W = 79, p = 0.0005; fig. 3b). The three outliers with the lowest residuals for the haplorhine group represent the only nocturnal haplorhine taxa (Aotus and Tarsius). However, strepsirrhines do not have higher residual acuity than other nonprimate mammals (Wilcoxon: W = 270, p = 0.142). Due to their derived acuity relative to eye size, haplorhines were excluded from all ecological analyses.
Ecological Influences on Variation in Visual Acuity
Nonparametric Tests
The effects of ecological variables on raw visual acuity are shown in figure 4. Visual acuity varies significantly across diel activity patterns (fig. 4a; Kruskal-Wallis: χ2(2) = 13.23, p = 0.001). Both diurnal and cathemeral species exhibit higher acuity than nocturnal species (diurnal vs. nocturnal Wilcoxon: W = 112.5, p = 0.001; cathemeral vs. nocturnal W = 127, p = 0.003). However, visual acuity in cathemeral and diurnal species does not significantly differ (Wilcoxon: W = 68, p = 0.086). While we found that active predators have higher median visual acuity than herbivores (fig. 2b), these differences were not statistically significant (Wilcoxon: W = 40.5, p = 0.241). Finally, we found that MRS is significantly positively correlated with visual acuity (fig. 4c; Spearman: S = 56.6, r = 0.80, p = 0.002).
Ecological factors potentially influencing visual acuity for the behavioral acuity dataset. a Diel activity pattern. Outliers: nocturnal galagos (Galago senegalensis, Otolemur crassicaudatus), cathemeral horse (Equus caballus) and diurnal camel (Camelus bactrianus). b Diet. Outlier: E. caballus (herbivore). c MRS. All analyses exclude haplorhines, semi-aquatic and fossorial species. Dietary analyses exclude microchiropteran bats. Boxplots are quartile, with whiskers representing highest and lowest values that are not outliers (closed circles; defined as >1.5 times the interquartile range).
Ecological factors potentially influencing visual acuity for the behavioral acuity dataset. a Diel activity pattern. Outliers: nocturnal galagos (Galago senegalensis, Otolemur crassicaudatus), cathemeral horse (Equus caballus) and diurnal camel (Camelus bactrianus). b Diet. Outlier: E. caballus (herbivore). c MRS. All analyses exclude haplorhines, semi-aquatic and fossorial species. Dietary analyses exclude microchiropteran bats. Boxplots are quartile, with whiskers representing highest and lowest values that are not outliers (closed circles; defined as >1.5 times the interquartile range).
The effects of ecology on residual visual acuity (i.e. visual acuity relative to eye length) are shown in figure 5. These residual analyses indicate that only diel activity pattern (fig. 5a) and diet (fig. 5b) have a significant influence on acuity once phylogeny and interspecific variation in eye length are statistically controlled. As expected, residual acuity varies by activity pattern (Kruskal-Wallis: χ2(2) = 7.02, p = 0.03), with diurnal species exhibiting significantly higher residual acuity than nocturnal species (Wilcoxon: W = 102, p = 0.006). Cathemeral species have significantly higher residual acuity than nocturnal species (Wilcoxon: W = 111, p = 0.033), but do not differ from diurnal taxa (W = 59, p = 0.251). Residual acuity also varies with diet, with active predators having significantly higher residual acuity than herbivores (Wilcoxon: W = 55, p = 0.014). Finally, in contrast to the findings for raw acuity, residual acuity is not significantly correlated with MRS (fig. 5c; Spearman: S = 250, r = 0.13, p = 0.70).
Ecological factors potentially influencing residual acuity for the behavioral acuity dataset. Residual acuity calculated from the behavioral PGLS regression. a Diel activity pattern. b Diet. Outlier: northern quoll (Dasyurus hallucatus; active predator). c MRS. All analyses exclude haplorhines, semi-aquatic and fossorial species. Dietary analyses exclude microchiropteran bats. Boxplots are quartile, with whiskers representing highest and lowest values that are not outliers (closed circles; defined as >1.5 times the interquartile range).
Ecological factors potentially influencing residual acuity for the behavioral acuity dataset. Residual acuity calculated from the behavioral PGLS regression. a Diel activity pattern. b Diet. Outlier: northern quoll (Dasyurus hallucatus; active predator). c MRS. All analyses exclude haplorhines, semi-aquatic and fossorial species. Dietary analyses exclude microchiropteran bats. Boxplots are quartile, with whiskers representing highest and lowest values that are not outliers (closed circles; defined as >1.5 times the interquartile range).
PGLS Multivariate Models
The results of all PGLS multivariate models are shown in table 2. The PGLS multivariate models that incorporate diel activity pattern and diet closely mirror our findings for residual acuity. These multivariate models demonstrate that both activity pattern and diet have a significant effect on visual acuity that is independent of eye length (table 2). Inclusion of activity pattern as a co-predictor variable results in a model that fits the data significantly better than the null model as measured by ANOVA (F2 = 3.95, p = 0.03) and increases the explanatory power of the model by ∼3.8%. As with the residual analyses, diurnal and cathemeral species have significantly higher acuity relative to eye size than nocturnal species (table 2).
The results of the multivariate models for diet suggest that active predators have significantly higher acuity relative to eye size compared to herbivorous species (table 2). The inclusion of diet as a co-predictor significantly improves the fit of the models to the data (ANOVA: F1 = 5.57, p = 0.033). Including diet as a co-predictor increases the explanatory power of the multivariate model by 5.19%.
In contrast to the results for diel activity pattern and diet, multivariate models show no significant effect of MRS on visual acuity once the effect of eye size has been taken into account (table 2). Furthermore, adding MRS to the null model does not improve the model fit (ANOVA: F1 = 1.325, p = 0.279) and only increases the explanatory power of the model by 0.8%. This result is consistent with our finding that MRS has no significant effect on residual acuity in nonparametric analyses.
Discussion
This study provides the first phylogenetically controlled analysis of the influence of eye size on visual acuity in a broad comparative sample of mammals. Our results show that visual acuity is strongly positively correlated with eye length, and that eye length alone can explain a substantial proportion of the interspecific variance in visual acuity (∼35% based on the behavioral acuity sample, ∼50% based on the combined acuity sample). In other words, mammals with absolutely larger eyes tend to have higher visual acuity than mammals with absolutely smaller eyes. This result is consistent with expectations based on optical considerations [Walls, 1942; Kirschfeld, 1976; Hughes, 1977; Pettigrew et al., 1988] and previous findings based on smaller comparative samples [e.g. Kiltie, 2000; Heesy and Hall, 2010]. Together, these results suggest that having absolutely larger eyes facilitates more detailed sampling of an absolutely larger retinal image by the photoreceptor mosaic. Although eye length is one of the variables that is often used to calculate anatomical estimates of visual acuity [e.g. Hughes, 1977; Pettigrew et al., 1988], we also found that the relationship between acuity and eye length does not substantially change depending on whether acuity is measured anatomically or behaviorally [Kiltie, 2000].
Our results provide support for the hypothesis that one of the proximate factors influencing the evolution of eye size in mammals is selection for increased visual acuity. For example, the large eyes of mammals with fast running speeds have been suggested to represent adaptations for increased visual acuity in order to avoid collisions with obstacles in the environment [Heard-Booth and Kirk, 2012]. Similarly, the large eyes of some mammalian taxa (e.g. felid carnivores, tarsiers and owl monkeys) are suggested to be the product of selection to increase visual acuity without compromising visual sensitivity at night [Ross, 2000; Kirk and Kay, 2004; Ross and Kirk, 2007]. Here we confirm a key component of these adaptive scenarios by showing that larger eyes are associated with higher visual acuity, suggesting that the two factors often evolve in tandem. Although our data provide no direct evidence that relaxed selection on visual acuity might lead to decreases in eye size, such an expectation is reasonable because eyes are metabolically expensive to grow and maintain [Schmidt et al., 2003; Niven and Laughlin, 2008]. Indeed, genetic evidence from cave-dwelling fish suggests that selection (rather than drift) may act to decrease eye size when vision is not necessary [Protas et al., 2007].
Ecology and Mammalian Visual Acuity
The fact that eye length explains only part of the interspecific variation in mammalian visual acuity is not surprising given the large number of other anatomical factors that are known to influence acuity [Walls, 1942; Hughes, 1977; Arrese et al., 1999; Ross, 2000; Kirk and Kay, 2004]. The configuration of the dioptric apparatus influences visual acuity through its effect on retinal image size, while refractive errors, pupil area and the density of retinal ganglion cells may each set an upper limit on the maximum acuity that can be attained in any mammal eye [Walls, 1942; Hughes, 1977; Land and Nilsson, 2012]. In addition to these proximate anatomical determinants of acuity, most of the ultimate evolutionary factors that select for differences in visual acuity between species are ecological [Walls, 1942; Hughes, 1977; Land and Nilsson, 2012]. In this context, our results provide strong evidence that diel activity pattern and diet are important selective factors influencing interspecific variation in mammalian visual acuity.
Diel activity pattern had a significant effect on raw visual acuity in our comparative sample, with diurnal and cathemeral species having higher raw acuity than nocturnal species. This result is partly expected because day-active mammals differ from nocturnal mammals in having eye morphology and retinal anatomy that supports higher acuity [Walls, 1942; Hughes, 1977; Pettigrew et al., 1988; Kirk, 2006b; Heesy and Hall, 2010; Hall et al., 2012; Land and Nilsson, 2012]. However, contrary to expectation that cathemeral species should exhibit visual adaptations that are intermediate between those of diurnal and nocturnal species [Walls, 1942; Kay and Kirk, 2000; Kirk, 2006b; Veilleux and Kirk, 2009], the diurnal and cathemeral mammals in our sample did not significantly differ in raw acuity. This result is probably due to the fact that many of the largest-bodied species in our sample are cathemeral (e.g. giraffe, horse and rhinoceros; online suppl. table 1). Eye size in mammals is highly positively correlated with body size [Ross and Kirk, 2007], and thus cathemeral taxa also have the largest eyes on our sample (online suppl. fig. 1a). These larger eyes of cathemeral species probably offset increased retinal summation and relatively shorter PND compared to the diurnal sample, leading to comparable raw acuity in the two groups.
After controlling for differences in eye length, residual acuity analyses and PGLS multivariate models both found that diurnal and cathemeral mammals have the highest visual acuity in our comparative sample. In other words, when comparisons are made between species of similar absolute eye size, diurnal and cathemeral taxa have significantly higher visual acuity than nocturnal taxa. Although diurnal and cathemeral species do not significantly differ in residual acuity, cathemeral species tend to have residual acuity values intermediate between those of diurnal and nocturnal species (fig. 5a). This variation in acuity independent of the observed effect of eye size is likely achieved through adaptations of the dioptric apparatus (to increase retinal image size) and/or retina (to increase sampling density), and is supported by a wealth of comparative data on mammalian eye morphology and retinal anatomy. Diurnal mammals often exhibit smaller relative cornea and lens sizes, lower rod:cone ratios, and lower retinal summation than nocturnal species (all of which tend to increase acuity) [Walls, 1942; Hughes, 1977; Arrese et al., 1999; Kay and Kirk, 2000; Ross, 2000; Kirk, 2004; Kirk and Kay, 2004; Silveira, 2004; Peichl, 2005; Kirk, 2006b; Ross and Kirk, 2007; Hall et al., 2012]. Furthermore, diurnal haplorhines have significantly higher acuity than other mammals due to highly derived retinal specializations (e.g. an all-cone fovea with no retinal summation) and eye morphologies (e.g. very small corneas relative to eye sizes) [Kay and Kirk, 2000; Ross, 2000; Kirk, 2004; Kirk and Kay, 2004; Kirk, 2006a, b; Ross and Kirk, 2007; Hall et al., 2012].
We also found that diet had a significant effect on visual acuity after statistically controlling for eye length. In both residual analyses and PGLS multivariate models, active predators had higher visual acuity than herbivores. These results suggest that predatory mammals that rely partly on vision to catch moving prey tend to have retinas that support higher visual resolution than the retinas of herbivorous mammals. While few studies have examined the effects of diet on mammalian eye morphology and retinal anatomy, more faunivorous mammals generally exhibit lower rod:cone ratios, higher ganglion cell densities and more convergent orbits (reflecting greater binocular field overlap) than herbivorous species [Peichl et al., 2004; Tetreault et al., 2004; Heesy, 2008]. Each of these factors should lead to increased acuity in faunivores if eye size and morphology is held constant. However, we did not find a significant difference in raw visual acuity between the two dietary groups. As with our results for cathemeral taxa, this finding is not entirely surprising because our herbivorous group includes many large-bodied and large-eyed species (online suppl. fig. 1b; online suppl. table 1)1.
Our raw acuity results support the expectation that species with faster MRS should exhibit higher visual acuity than species with slower MRS (fig. 4c) [Walls, 1942; Hughes, 1977; Heard-Booth and Kirk, 2012]. This finding could be explained as the result of selection for increased visual acuity in fast running mammals in order to avoid collisions with environmental obstacles [Walls, 1942; Hughes, 1977; Heard-Booth and Kirk, 2012]. However, our results also demonstrate that when eye length is held constant, visual acuity is not significantly related to MRS. The quality of data on maximum speed of locomotion can vary considerably according to the methodologies employed by different authors, and thus interpretations of these results should be treated with caution [Garland, 1983]. Nonetheless, our findings suggest that while MRS may influence interspecific variation in eye length in mammals, it is not associated with additional retinal or dioptric adaptations for increased acuity. If this interpretation is correct and fast-running mammals have large eyes but lack clear retinal adaptations for enhanced visual acuity, then it suggests that the observed relationship between eye size and MRS in mammals [Heard-Booth and Kirk, 2012] may be the result of selection favoring factors other than increased maximum visual acuity. For example, increased eye size may have important consequences for retinal circuits related to the perception of motion and optical flow within the retinal periphery. At a minimum, our findings suggest that current interpretations of the relationship between eye size and maximum speed of locomotion require further refinement based on analyses of additional data [see also Hall and Heesy, 2011].
Finally, it is also worth noting that anatomical evidence suggests that additional ecological factors not considered here may further influence interspecific variation in mammalian visual acuity. For example, Veilleux and Lewis [2011] recently found that mammalian eye shape varied predictably with ambient light intensity in different habitat types. In particular, mammals from more open habitats exhibited smaller relative cornea sizes compared to species from closed canopy forests. We would thus predict that, controlling for activity pattern, more open habitat-dwelling mammals exhibit higher visual acuity. Accordingly, it may not be coincidental that the 3 nonprimate mammals with the highest acuity in the present analysis (i.e. horses, cheetahs, giraffes) typically inhabit more open environments [Nowak, 1999].
Conclusions
Interspecific differences in mammalian eye size have predictable consequences for visual acuity. Eye length and visual acuity are significantly positively correlated in mammals, and thus species with absolutely large eyes tend to have higher visual acuity than species with absolutely small eyes. Some nocturnal bats and rodents have lower visual acuity than expected for their eye sizes, while diurnal anthropoid primates have high acuity for their eye sizes. When differences between species in absolute eye size are statistically controlled, diurnal species have higher visual acuity than nocturnal species, and active predators have higher visual acuity than herbivores. These results suggest that diurnal species and active predators both demonstrate dioptric and/or retinal adaptations for enhanced acuity. Although species with high MRS also tend to have absolutely high visual acuity compared to species with lower MRS, there is no relationship between MRS and visual acuity once the effect of eye length is held constant. This finding suggests that fast-running mammals may lack specific retinal adaptations for enhanced acuity. If so, current functional explanations for the observed relationship between eye size and MRS in mammals may require revision.
Acknowledgements
We would like to thank Andrew Barr, Amber Heard-Booth, Margaret Hall, Addison Kemp, Charlie Nunn and Clara Scarry for helpful discussions. We also thank three anonymous reviewers for providing useful comments for the revision of this paper.
References
Footnotes
Median body mass for the active predators in our dataset was 0.83 kg (range 0.02-64.23), while that for herbivores it was 6.73 kg (range 0.009-2,420.33).