Abstract
Objectives: Bone trauma is a common occurrence in human skeletal remains. Macroscopic and imaging scrutiny is the approach most currently used to analyze and describe trauma. Nevertheless, this line of inquiry may not be sufficient to accurately identify the type of traumatic lesion and the associated degree of bone healing. To test the usefulness of histology in the examination of bone healing biology, we used an integrative approach that combines gross inspection and microscopy. Materials and Methods: Six bone samples belonging to 5 adult individuals with signs of bone trauma were collected from the Human Identified Skeletal Collection from the Museu Bocage (Lisbon, Portugal). Previous to sampling, the lesions were described according to their location, morphology, and healing status. After sampling, the bone specimens were prepared for plane light and polarized light analysis. Results: The histological analysis was pivotal: (1) to differentiate between types of traumatic lesions; (2) to ascertain the posttraumatic interval, and (3) to diagnose other associated pathological conditions. Conclusion: The outer surface of a bone lesion may not give a complete picture of the biology of the tissue's response. Accordingly, microscopic analysis is essential to differentiate, characterize, and classify trauma signs.
Introduction
Injury constitutes one of the major causes of death and disability worldwide and a source of health and social concern with regard to prevention and treatment [1]. As nowadays, injury has also played a significant role in past human societies, a role that can be accessed through a careful analysis of the skeletal remains [2]. Bone injury, resulting from interpersonal violence or associated with the hazards of daily life, is frequently reported in the paleopathological literature as a useful tool to decipher past behaviors and health conditions [3,4,5,6,7,8,9,10,11,12,13,14]. The prevalence and distribution of fractures constitute the marker most commonly investigated [15,16,17,18,19,20]. The study of fractures in the skeletonized remains relies on the macroscopic and radiological identification of signs of bone healing [21,22,23] and allied complications such as bone shortening, deformity, infection, and pseudarthrosis, among others [21,23,24,25]. As in the clinical context, imaging analysis is considered the best line of inquiry for diagnosing and classifying fractures [26]. Nevertheless, its application may be restricted due to the unavailability of equipment or by the presence of diagenetic bone changes that may bias the radiological interpretation [23,27]. As a result, the visual inspection of complete or partial callus formation [10,15] and/or angular deformities [10] constitutes the methodological approach most often used. What about paleohistology? What information concerning bone healing can be gained from the application of histological techniques?
The paleohistopathological analysis of skeletal remains offers a glimpse into the products of cellular activity such as excessive tissue mineralization, signs of abnormal bone resorption, and residual organics associated with past diseases [28,29]. Furthermore, it provides a wide range of information with regard to microstructural changes that bone undergoes during burial, and that can affect disease diagnosis [30]. Wright and Yoder [31] stated that the application of histological methods may be especially important for examining the degrees of bone healing, as well as for identifying traces of disease in cases where little bone response occurred prior to death. This assertion is equally shared by Ragsdale and Lehmer [32]: if cells are the effectors of bone changes, understanding their dynamic interaction is a necessary step for the differential diagnosis.
Using two methodological approaches that compare macroscopic and histological observations, this study aims: (1) to describe the macro- and microstructure of the bone callus; (2) to ascertain the biology of bone healing and the posttraumatic survival interval, and (3) to discuss diagnoses by identifying other conditions that might have caused the lesions observed.
Materials and Methods
Five adult individuals with multiple trauma evidence and housed at the Human Identified Skeletal Collection from the Museu Bocage (Lisbon, Portugal) were chosen for analysis [33]. However, only 6 bone calluses on the ribs and long bones were targeted for sampling (table 1). For skeleton 1,196 (Sk. 1,196), permission was granted to take 2 samples. Due to the invasive nature of the histological procedures, bone sampling was adjusted in order to avoid needless damage to the skeletons. For example, when possible the samples were taken close to damaged areas or in bones showing postmortem breakage. Prior to bone sampling, fractures were classified according to their location, stage of healing and/or presence of bone malalignment. Only lesions that were healing (presence of woven bone near the fracture edges or surrounding the primary callus) or had a healed appearance (presence of sclerotic or dense bone) were considered [22]. After sampling, the bone specimens were prepared for histological analysis [34] as the follows: (1) cleaning and dehydration in water and ethyl alcohol (95%), respectively; (2) embedding in epoxy resin; (3) sectioning using a slow speed saw; (4) grinding using graded sandpaper discs, and an abrasive slurry of aluminum oxidate; (5) dehydration in ethyl alcohol (95%), followed by immersion in xylene (vacuum chamber) and (6) microscopic analysis under plane and polarized light. The qualitative description of the callus histomorphology considered the bone tissue involved (periosteum, cortex, or endosteum), the type of bone response observed (resorption, formation, or both), the type of new bone produced (woven, lamellar or both), and the prevalence of osteocyte lacunae.
Results
Macroscopic Study of the Degree of Bone Healing
A total of 42 consolidated and unconsolidated bone calluses were observed in the 5 adult individuals considered for analysis (table 2). Of these, 35.7% (15/42) were seen in the adult male Sk. 1,138 (86 years old), 30.9% (13/42) in the adult female Sk. 119 (64 years old), and 23.8% (10/42) in the adult female Sk. 1,196 (75 years old). With the exception of Sk. 54, all the remaining individuals exhibited multiple signs of bone trauma. The majority of the bone calluses were recorded on ribs, mainly in those from the lower segment (R7-R10). For example, 15 bone calluses, 7 of them unconsolidated, were observed in 8 right ribs and in one left rib of the Sk. 1,138 individual. This individual also showed a combination of healed and unconsolidated fractures in 5 ribs. The other bone calluses were observed in the radii (Sk. 1,196), tibia (Sk. 54), and fibula (Sk. 198).
Distribution of the evidence of bone trauma in the individuals analyzed by bone element, anatomic location, and rate of consolidation
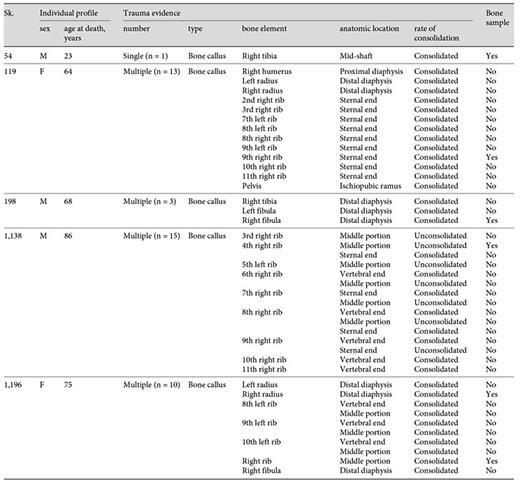
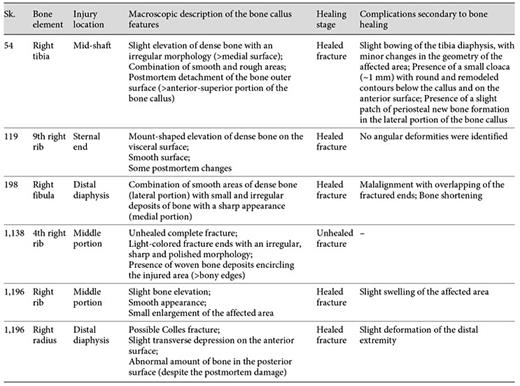
Of the 6 bone elements selected for histological analysis, 5 presented bone calluses with a consolidated and healed appearance (table 3). That is, the calluses appeared slightly elevated from the bone surface and presented a dense and smooth outer shell. Only the 4th right rib of the Sk. 1,138 individual showed an unconsolidated callus. Depending on the bone element, the callus morphology ranged from a sharp outlook to a mount-shaped or round relief (e.g. Sk. 119; fig. 1a-d). In 2 individuals (Sk. 54 and Sk. 198), the fracture had introduced slight structural changes in the bone architecture (fig. 2a-d, 3a-c). In the case of the Sk. 198 right fibula, an inefficient stabilization had caused a malalignment of the shaft with an overlap of the broken ends and subsequent bone shortening. In addition to some structural changes, a small cloaca (∼1 mm) with remodeled contours (anterior portion) and a patch of periosteal new bone formation (lateral portion) were observed in the Sk. 54 tibia bone callus. Healed fractures were seen in the ribs and at the distal extremity of the Sk. 1,196 radii, causing a slight epiphysis malalignment (fig. 4a-b2). In the unhealed rib fracture (Sk. 1,138), the broken edges presented an irregular, smooth, and polished morphology. Surrounding the affected area, deposits of periosteal new bone were seen detached from the surface. No ‘movable' joint-like structure was identified at the fractured ends (fig. 5a-d).
Evaluation of the macroscopic features of the bone callus by individual, bone element, location, and healing stage
a Sternal end of the 9th right rib of an adult female (Sk. 119, 64 years old) who died of bronchopneumonia. b Detail of the area sampled for analysis showing a slight round elevation (white arrow). c Rib section collected for histological analysis. d Detail of the bone sample after slide preparation, in which is visible an accumulation of bone on the visceral surface.
a Sternal end of the 9th right rib of an adult female (Sk. 119, 64 years old) who died of bronchopneumonia. b Detail of the area sampled for analysis showing a slight round elevation (white arrow). c Rib section collected for histological analysis. d Detail of the bone sample after slide preparation, in which is visible an accumulation of bone on the visceral surface.
Tibiae from an adult male (Sk. 54, 23 years old) who died of pulmonary tuberculosis. a Right tibia showing a consolidated callus on the middle of the diaphysis. Note the presence of slight structural changes of the shaft when compared with the unaffected left tibia. b Detail of the bone callus showing an expanded and smooth surface (medial face). The black asterisk indicates the area sampled for histological analysis. c Magnification of the anterior-lateral portion of the bone callus exhibiting some postmortem detachment of the outer shell of the callus and a small cloaca below the callus. d Bone sample collected for histological analysis, before (left) and after slide preparation (right).
Tibiae from an adult male (Sk. 54, 23 years old) who died of pulmonary tuberculosis. a Right tibia showing a consolidated callus on the middle of the diaphysis. Note the presence of slight structural changes of the shaft when compared with the unaffected left tibia. b Detail of the bone callus showing an expanded and smooth surface (medial face). The black asterisk indicates the area sampled for histological analysis. c Magnification of the anterior-lateral portion of the bone callus exhibiting some postmortem detachment of the outer shell of the callus and a small cloaca below the callus. d Bone sample collected for histological analysis, before (left) and after slide preparation (right).
a Right fibula of an adult male (Sk. 198, 68 years old) who died of urinary sepsis. b Detail of the bone callus exhibiting a consolidated but irregular morphology. Note the overlapping of the fractured ends. c Magnification of the sample collected for histological analysis, before (left) and after slide preparation (right).
a Right fibula of an adult male (Sk. 198, 68 years old) who died of urinary sepsis. b Detail of the bone callus exhibiting a consolidated but irregular morphology. Note the overlapping of the fractured ends. c Magnification of the sample collected for histological analysis, before (left) and after slide preparation (right).
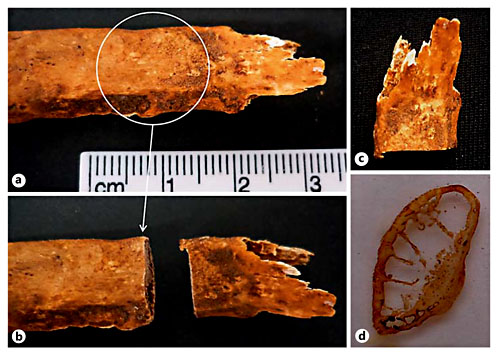
a Radii from Sk. 1,196, a 75-year-old female who died of arteriosclerosis showing trauma evidence compatible with a Colles fracture. a1 Detail of the bone callus of the right radius exhibiting an abnormal elevation. Note the severe postmortem damage (dorsal view). a2 Anterior view of the affected radius. a3 Magnification of the bone sample collected for histological analysis, before (left) and after (right) slide preparation. The black asterisk indicates the surface sampled for histological analysis. b Right rib from the same individual with a consolidated callus (white arrow). b1 Visceral surface of the above-mentioned sample highlighting the smooth surface of the callus (white arrow). b2 Detail of the sample collected after slide preparation.
a Radii from Sk. 1,196, a 75-year-old female who died of arteriosclerosis showing trauma evidence compatible with a Colles fracture. a1 Detail of the bone callus of the right radius exhibiting an abnormal elevation. Note the severe postmortem damage (dorsal view). a2 Anterior view of the affected radius. a3 Magnification of the bone sample collected for histological analysis, before (left) and after (right) slide preparation. The black asterisk indicates the surface sampled for histological analysis. b Right rib from the same individual with a consolidated callus (white arrow). b1 Visceral surface of the above-mentioned sample highlighting the smooth surface of the callus (white arrow). b2 Detail of the sample collected after slide preparation.
a The 4th right rib of the adult male (Sk. 1,138, 86 years old) who died of bronchopneumonia, revealing a complete, unconsolidated fracture in the sternal portion. b Magnification of the fractured ends showing a sharp morphology and the presence of a rim of newly built bone (dorsal view). c Visceral surface of the rib showing the fractured edges. d Detail of the previous image showing the new bone foci on the sample (left) and the slide prepared for histological analysis (right).
a The 4th right rib of the adult male (Sk. 1,138, 86 years old) who died of bronchopneumonia, revealing a complete, unconsolidated fracture in the sternal portion. b Magnification of the fractured ends showing a sharp morphology and the presence of a rim of newly built bone (dorsal view). c Visceral surface of the rib showing the fractured edges. d Detail of the previous image showing the new bone foci on the sample (left) and the slide prepared for histological analysis (right).
Histomorphology of the Bone Callus
The histological analysis of the 6 bone samples showed a well-preserved bone microanatomy with good bone birefringence. Some structural differences were found when the bone calluses were compared (table 4).
Evaluation of the histological features of the bone callus by individual, sample, healing stage, and type of trauma diagnosis
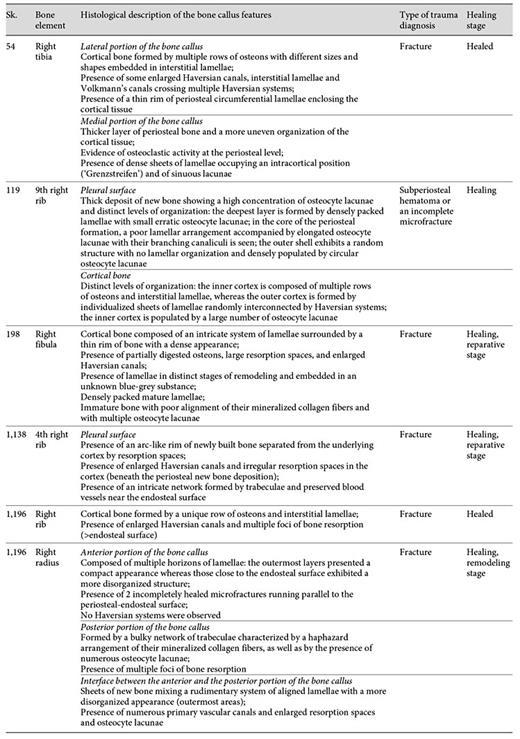
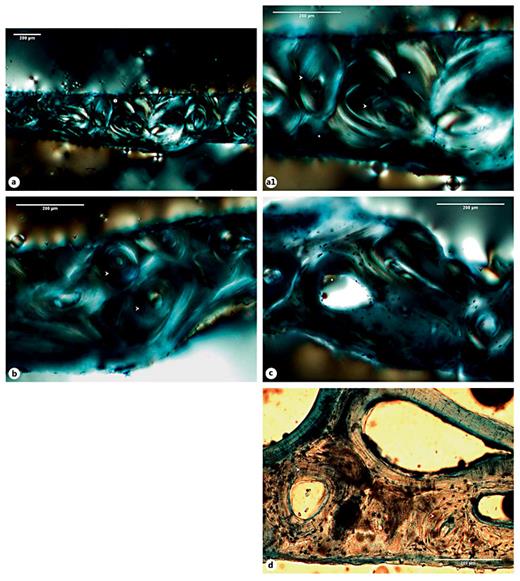
Of the 5 bone samples with an apparently healed callus, only 2 (Sk. 54 and 1,196 - rib sample) presented a truly mature and remodeled microstructure. For example, in the lateral portion of the Sk. 54 right tibia callus, a haphazard arrangement of Haversian systems, interstitial lamellae, and enlarged osteon canals crisscrossed by Volkmann's canals was observed. Discontinuous rows of intracortical lamellae, which resemble ‘Grenzstreifen' [35,36,37] were seen separating the inner cortical bone from the secondary compact bone. At some points, erratic resorption lacunae, or sinuous lacunae [37], parallel to the intracortical lamellae were also noticed. In the medial portion of the bone callus, a profusion of randomly organized lamellae in different stages of maturation was observed. In this area, a more chaotic structure formed by osteons, Howship's lacunae and remnants of densely packed lamellae were seen. The periosteal microarchitecture ranged from a thin rim of bone to a denser and/or ruffled surface showing, at some points, irregular resorption spaces (fig. 6a-e).
a Microscopic view of the Sk. 54 right tibia sample showing the cortical tissue formed by osteons (white arrowheads) intersected, at some points by Volkmann's canals (black arrowheads). The periosteal surface was composed of a thin rim of bone (white asterisks). b Segment formed by osteons, intracortical lamellae (circle 1), and resorption spaces (circle 2). b1 Detail of the intracortical lamellae (or ‘Grenzstreifen'; black arrowheads) separating rows of osteons (white arrowheads). b2 Another magnification showing some resorption lacunae (or sinuous lacunae; white asterisk) in the intracortical lamellae (black arrowheads) and at the periosteal surface (white asterisk). Note the presence of osteons with enlarged Haversian canal (white arrowhead). c Segment showing a haphazard cortical microstructure formed by osteons (white arrowheads), interstitial lamellae (white asterisks), and compact intracortical lamellae (black arrowheads). d Thick layer of dense bone at the periosteal surface (black asterisks) connecting the cortical tissue formed by different sized osteons (white arrowheads). e Another segment revealing erratic osteons (white arrowheads) surrounded by elongated intracortical lamellae (black arrowheads). Polarized light. Magnification ×40; ×100.
a Microscopic view of the Sk. 54 right tibia sample showing the cortical tissue formed by osteons (white arrowheads) intersected, at some points by Volkmann's canals (black arrowheads). The periosteal surface was composed of a thin rim of bone (white asterisks). b Segment formed by osteons, intracortical lamellae (circle 1), and resorption spaces (circle 2). b1 Detail of the intracortical lamellae (or ‘Grenzstreifen'; black arrowheads) separating rows of osteons (white arrowheads). b2 Another magnification showing some resorption lacunae (or sinuous lacunae; white asterisk) in the intracortical lamellae (black arrowheads) and at the periosteal surface (white asterisk). Note the presence of osteons with enlarged Haversian canal (white arrowhead). c Segment showing a haphazard cortical microstructure formed by osteons (white arrowheads), interstitial lamellae (white asterisks), and compact intracortical lamellae (black arrowheads). d Thick layer of dense bone at the periosteal surface (black asterisks) connecting the cortical tissue formed by different sized osteons (white arrowheads). e Another segment revealing erratic osteons (white arrowheads) surrounded by elongated intracortical lamellae (black arrowheads). Polarized light. Magnification ×40; ×100.
A remodeled callus microstructure characterized by well-defined osteons and interstitial lamellae was also identified in the right rib sample retrieved from Sk. 1,196. In this sample, multiple bays of bone resorption were observed in the endosteal and periosteal surfaces. At some points, larger areas of bone resorption enclosed by thin layers of bone lamellae were seen, in addition to enlarged Haversian canals and numerous osteocyte lacunae (fig. 7a-d). In contrast with the above-mentioned case, the sample retrieved from the right radius of the same Sk. 1,196 individual showed a more immature microstructure. For example, an intricate network of trabeculae, some of them with signs of bone resorption, was observed in the posterior side of the bone callus. The typical structure of a mature cortical tissue was recorded as absent from the core of the bone callus, as well as from the opposite anterior surface. That is, no clearly defined osteons, interstitial lamellae, and Haversian canals were observed. Instead, the anterior portion appeared to be formed by horizons of lamellar bone pinpointed by a high density of osteocyte lacunae, which suggests distinct levels of bone deposition. Irregular lines running alongside the bone lamellae were also seen. Finally, a pattern of disorganized lamellae and immature bone populated by osteocyte lacunae and separated by irregular areas of bone resorption and discrete Haversian canals was seen in the interface between the anterior and the posterior surfaces of the bone callus (fig. 8a-e1). The most striking example of an immature callus microstructure came from the Sk. 198 sample. In spite of the healed macroscopic appearance, the histological study revealed a cortical tissue formed by an intricate system of lamellae, comparable to trabeculae, in which multiple branches and islands of well-preserved lamellae connecting partially digested osteons were observed. A combination of mature lamellae with more immature bone populated by multiple osteocyte lacunae and large resorption spaces was also noticed. At the periosteal level, a rim of lamellae in distinct stages of maturation was seen bordering the outer surface of the bone (fig. 9a-d).
a Micrograph of the right rib sample of the SK. 1,196 individual exhibiting the cortical tissue (circle 1). a1 Detail of the previous figure showing a thin row of osteons (white arrowheads) and interstitial lamellae (white asterisks). b Another segment revealing bays of bone resorption in the endosteal and periosteal surfaces (black arrowheads) and some intact cortical osteons (white arrowheads). c Segment showing a larger area of cortical (white asterisk) and endosteal (black arrowheads) bone resorption. d Illustration of a segment under plane light exhibiting a row of osteons (white arrowheads) with enlarged Haversian canals and numerous osteocyte lacunae. Polarized light. Magnification ×40; ×100.
a Micrograph of the right rib sample of the SK. 1,196 individual exhibiting the cortical tissue (circle 1). a1 Detail of the previous figure showing a thin row of osteons (white arrowheads) and interstitial lamellae (white asterisks). b Another segment revealing bays of bone resorption in the endosteal and periosteal surfaces (black arrowheads) and some intact cortical osteons (white arrowheads). c Segment showing a larger area of cortical (white asterisk) and endosteal (black arrowheads) bone resorption. d Illustration of a segment under plane light exhibiting a row of osteons (white arrowheads) with enlarged Haversian canals and numerous osteocyte lacunae. Polarized light. Magnification ×40; ×100.
a Micrograph of the posterior portion of the SK. 1,196 right radius bone callus showing scattered trabeculae erased by foci of bone resorption (white arrowheads). b Illustration of the anterior portion of the callus revealing lamellar bone apposition with variable density and organization (white asterisks). c Another view revealing distinct layers of lamellae (white asterisks) separated by longitudinal lines (white arrowheads). d Area showing cortical lamellae mixed with more immature and disorganized forms of bone (white and black arrowheads), and spaces of bone resorption (white asterisks) and discrete vascular canals. e Segment exhibiting a haphazard arrangement of bone lamellae in distinct stages of maturation (white arrowheads), as well as enlarged resorption spaces (white asterisks). e1 Detail of the previous figure highlighting the orientation of the mineralized collagen fibers and the numerous osteocyte lacunae. Polarized light. Magnification ×40; ×100.
a Micrograph of the posterior portion of the SK. 1,196 right radius bone callus showing scattered trabeculae erased by foci of bone resorption (white arrowheads). b Illustration of the anterior portion of the callus revealing lamellar bone apposition with variable density and organization (white asterisks). c Another view revealing distinct layers of lamellae (white asterisks) separated by longitudinal lines (white arrowheads). d Area showing cortical lamellae mixed with more immature and disorganized forms of bone (white and black arrowheads), and spaces of bone resorption (white asterisks) and discrete vascular canals. e Segment exhibiting a haphazard arrangement of bone lamellae in distinct stages of maturation (white arrowheads), as well as enlarged resorption spaces (white asterisks). e1 Detail of the previous figure highlighting the orientation of the mineralized collagen fibers and the numerous osteocyte lacunae. Polarized light. Magnification ×40; ×100.
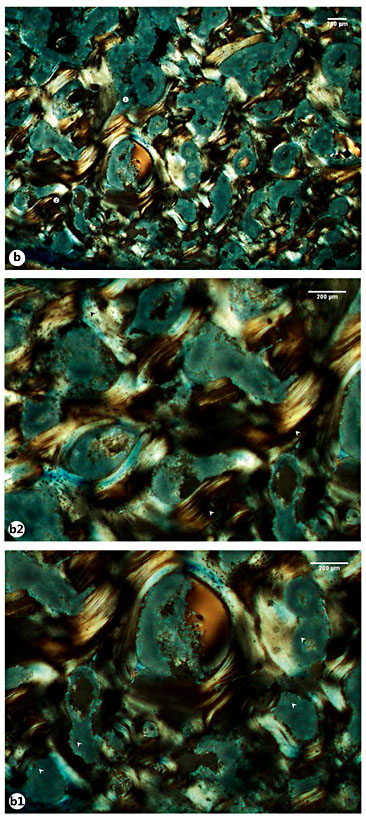
a Microscopic view of the Sk. 198 right fibula callus showing the cortical tissue formed by a disorganized net of bone lamellae and remnants of ancient Haversian systems (circle 1 and 2). a1, a2 Details of the previous image showing large bays of bone resorption being formed after digestion of previous osteons (white arrowheads). b Another view highlighting numerous areas of bone resorption. b1 Magnification of the previous image revealing the large and irregular areas of bone resorption (white arrowheads). b2 Another magnification showing mature (white arrowheads) and more recently formed lamellae with osteocyte lacunae (black arrowheads). c Bone segment combining densely packed lamellae on the bone surface (black arrowheads) and branches of disorganized lamellae with osteocyte lacunae in the innermost areas of the cortical bone (white star). d Another view showing the outer surface composed of lamellae with a haphazard arrangement (white star) and pinpointed by numerous osteocyte lacunae. Polarized light. Magnification ×40; ×100.
a Microscopic view of the Sk. 198 right fibula callus showing the cortical tissue formed by a disorganized net of bone lamellae and remnants of ancient Haversian systems (circle 1 and 2). a1, a2 Details of the previous image showing large bays of bone resorption being formed after digestion of previous osteons (white arrowheads). b Another view highlighting numerous areas of bone resorption. b1 Magnification of the previous image revealing the large and irregular areas of bone resorption (white arrowheads). b2 Another magnification showing mature (white arrowheads) and more recently formed lamellae with osteocyte lacunae (black arrowheads). c Bone segment combining densely packed lamellae on the bone surface (black arrowheads) and branches of disorganized lamellae with osteocyte lacunae in the innermost areas of the cortical bone (white star). d Another view showing the outer surface composed of lamellae with a haphazard arrangement (white star) and pinpointed by numerous osteocyte lacunae. Polarized light. Magnification ×40; ×100.
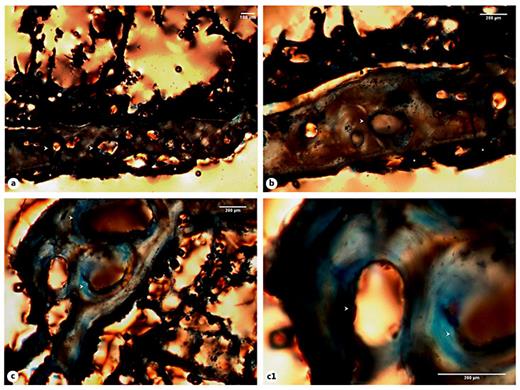
A complete unhealed fracture was observed on the 4th right rib of the adult male Sk. 1,138. In this particular case, the most revealing histological feature observed was the presence of an arc-like structure of new bone connected with the underlying cortex by bone pedestals. Intact endosteal and periosteal circumferential lamellae were also visible, as were remnants of blood vessels. In fact, a network formed by rib trabeculae and preserved blood vessels was observed on the endosteal surface. Enlarged Haversian canals and irregular resorption spaces were visible at the cortical level (fig. 10a-c1).
a Micrograph of the Sk. 1,138 rib cortical tissue presenting mature osteons and enlarged Haversian canals (white arrowheads) and a deposit of new bone with an arc-like microanatomy at periosteal level (white asterisks). Note the presence of rib trabeculae and preserved blood vessels (black arrowheads). b Another view pinpointing a major area of osteonal bone resorption (white arrowheads) and the newly built bone (white asterisks). c, c1 Bone segments showing massive foci of osteon resorption (white arrowheads). Polarized light. Magnification ×40; ×100.
a Micrograph of the Sk. 1,138 rib cortical tissue presenting mature osteons and enlarged Haversian canals (white arrowheads) and a deposit of new bone with an arc-like microanatomy at periosteal level (white asterisks). Note the presence of rib trabeculae and preserved blood vessels (black arrowheads). b Another view pinpointing a major area of osteonal bone resorption (white arrowheads) and the newly built bone (white asterisks). c, c1 Bone segments showing massive foci of osteon resorption (white arrowheads). Polarized light. Magnification ×40; ×100.
With regard to the Sk. 119 rib callus, no evidence of a cortical bone break was observed. Actually, the cortex exhibited a mature structure composed of numerous rows of osteons and interstitial lamellae. In the upper and lower edges of the pleural surface, a clear separation between the cortical bone and the patches of periosteal new bone was noticed. This level of microstructural organization was clearly distinguishable from the mesh-like pattern of interconnected lamellae and amorphous new bone formation noticed on the central portion of the pleural surface. Moreover, an accumulation of new bone in distinct stages of maturation was also observed. For instance, the deepest layers were formed by densely packed lamellae with small, elongated and erratic osteocyte lacunae, whereas the outermost ones were composed of a random structure with an immature appearance and were densely populated by circular osteocyte lacunae (fig. 11a-c1).
a Micrograph of the Sk. 119 right rib showing the cortical tissue composed of mature osteons (white arrowheads) and interstitial lamellae. A clear separation between the periosteal circumferential lamellae and a more disorganized periosteal new bone formation (white asterisk) is visible. b Segment exhibiting several rows of osteons (white arrowhead) intersected by sheets of lamellar bone with variable density (black arrowheads). Note the presence of a lumpy deposit of periosteal new bone (white asterisks). c Periosteal new bone formation exhibiting several degrees of bone organization (black arrowheads; circle 1). Multiple osteons are visible at the cortical level (white arrowhead). c1 Detail of the previous figure showing the inner layers composed of well-defined lamellae with small and elongated osteocyte lacunae and the outmost layers with a general lack of bone organization and populated by large and rounded osteocyte lacunae. Polarized light. Magnification ×40; ×100.
a Micrograph of the Sk. 119 right rib showing the cortical tissue composed of mature osteons (white arrowheads) and interstitial lamellae. A clear separation between the periosteal circumferential lamellae and a more disorganized periosteal new bone formation (white asterisk) is visible. b Segment exhibiting several rows of osteons (white arrowhead) intersected by sheets of lamellar bone with variable density (black arrowheads). Note the presence of a lumpy deposit of periosteal new bone (white asterisks). c Periosteal new bone formation exhibiting several degrees of bone organization (black arrowheads; circle 1). Multiple osteons are visible at the cortical level (white arrowhead). c1 Detail of the previous figure showing the inner layers composed of well-defined lamellae with small and elongated osteocyte lacunae and the outmost layers with a general lack of bone organization and populated by large and rounded osteocyte lacunae. Polarized light. Magnification ×40; ×100.
Discussion
Apart from the macroscopic and radiological scrutiny, few paleopathological studies have applied histological techniques to characterize different types of injury lesions [38,39,40,41,42,43,44,45] or to understand the timings of posttraumatic healing [46,47,48,49]. The results of this exploratory investigation showed that gross inspection may be insufficient to describe and characterize trauma lesions or to understand the biology of disease.
Despite the morphology of the outer shell of the Sk. 119 rib callus, which resembles a consolidated fracture, the histological study showed an unremodeled architecture compatible with a subperiosteal hematoma eventually caused by periosteum detachment, bleeding, and new bone formation through activation of the osteogenesis process. Nevertheless, a case of an incomplete microfracture cannot be completely excluded from the present diagnosis. The microstructure of hematoma is variable, ranging from thin layers that resemble a slip-like cover to relatively short, bulky bone trabeculae with extensive bridging, arc-like formation, and/or multiple layers [35,36,37]. In the case under discussion, the presence of tissues in distinct stages of maturation: immature and more disorganized bone/isodiametric osteocyte lacunae (outer layers) and lamellar bone/flattened osteocyte lacunae (deepest layers) seem to indicate that the bone lesion was undergoing remodeling at the time of death. Little can be said about the elapsed time after hematoma formation; nevertheless, and during fracture repair, the remodeling of woven bone into longitudinally oriented lamellar bone is observed to occur 14-21 days after injury [48]. Independently of the diagnosis, the presence of 9 rib calluses (n = 6, right ribs; n = 3, left ribs; table 2) seems to indicate that this female was exposed to chest trauma that caused a minor tissue disruption in the 9th right rib.
With regard to the Sk. 198 right fibula callus, the mesh-like pattern observed seems to mirror the last phases of the reparative stage. The remodeling phase is the longest (it may require 6-9 years in adults) and aims to reestablish the skeletal integrity [50,51]. The reparative phase is characterized by the development of an organized fibrous mass [52] in a process that recapitulates the embryonic intramembranous and endochondral ossifications [53,54,55]. This soft or fibrous callus will bond the broken ends [24] and guarantee the mechanical stability of the injured area [56]. Other local changes include mineralization [50], degradation of the nonmineralized matrix, and the formation of new trabeculae, which compounds the primary bony callus [24]. In the study of bone callus morphogenesis, Gerstenfeld et al. [57] showed that during the endochondral process of fracture healing, the cortex and cartilage undergo resorption, being replaced by an inner supporting network of trabeculae that will stabilize the fracture. Ayoub et al. [58] also observed a characteristic histological picture characterized by islands of newly built bone surrounded by cartilage and interspersed, at some points, by lamellar bone. The reparative stage may last several weeks. In some cases, the bony callus may originate as early as the first week after injury [59]. Analyzing the morphology of mineralized bone calluses, Liu et al. [60] noticed that a microstructure composed of poorly organized tissue (woven bone) and well-aligned lamellae develops during the 2-9 weeks of healing.
In contrast with the aforementioned case, the histomorphology of the Sk. 54 bone callus is compatible with a mature and remodeled fracture. The presence of a cloaca and the observation of small patches of intracortical lamellae or ‘Grenzstreifen' confirm that this individual had developed a posttraumatic osteomyelitis. Albeit more common in specific infections characterized by slowly growing new bone formation (treponematoses) [35,36,37,61], ‘Grenzstreifen' may also occur in cases of nonspecific osteomyelitis [37]. In the case under discussion, the ‘Grenzstreifen' separates the original cortical bone affected by trauma from a secondary inflammatory process imposed by osteomyelitis.
Like osteomyelitis, pseudarthrosis is another severe fracture complication. It develops when the broken extremities fail to form a bony union, which may happen, for instance, by lack of immobilization [52,62]. The continuing mobility of the affected area may culminate in the formation of a pseudojoint associated with extensive callus formation [24,62]. In cases of fracture nonunion, pseudarthrosis may mimic the healing process, leading to misdiagnosis [63]. With regard to the unhealed Sk. 1,138 rib fracture, the absence of a ‘false joint' makes a diagnosis of pseudarthrosis improbable. Accordingly, the histological features observed (periosteal osteogenesis distant from the lesion edges and separable from the cortex and cortical osteoporosis) are more compatible with the reparative stage of the healing process, pointing to a possible posttraumatic survival interval of 7-14 days [47]. As mentioned previously, the Sk. 1,138 individual exhibited 7 unhealed fractures (in a total of 15 bone calluses) distributed through 9 ribs. Although acute chest trauma is a possible explanation for the lesions observed, one cannot put aside the eventual contributions of age and associated bone fragilities. In addition to age, numerous factors such as the type, location, and severity of the fracture, the stability of the fractured ends, or the adequacy of the vascular supply may affect the healing process [24,52]. Few conclusions can be drawn from the impact of the causes of death of the individuals on the duration of the healing process; nevertheless, the age, and possibly the health condition, may have played a role in some of the cases described, namely in the healing of the Sk. 1,196 calluses. In the radius, for instance, the presence of a callus with partially digested trabeculae and unremodeled lamellae seems to suggest the existence of an underlying condition. It appeared that the bone was continuously laid down in a lamellar fashion without being converted into secondary Haversian systems. Some metabolic disorders may reduce the bone turnover [64]. When this happens, there is more time for secondary mineralization to proceed; as a consequence, the bone tissue becomes hypermineralized and more brittle, requiring less energy to microdamage [65]. Aging also diminishes the ability of bone to repair, leading to osteopenia and, in severe cases, to osteoporosis [66]. In addition to the presence of fractures (Colles fracture, vertebrae, hip, and ribs), age-related features of osteoporosis may include increased resorption and/or marked coalescence of resorption spaces, mineralization defects, and microcracks [[66] and authors herein]. These changes were noticed in the radius and in the rib samples. In spite of being difficult to ascertain whether the fractures were predisposed by a metabolic disorder, it may be hypothesized that it was affecting the healing process. Through histological analysis, it was also possible to verify that Sk. 1,196 suffered multiple traumatic episodes; an early one was completely healed at the time of death (rib fracture), and the other was healing when the individual died (radius fracture).
Conclusion
In spite of the small number of samples, this study showed that histology is important for several reasons. Firstly, it is essential to accurately identify the stage of fracture healing, information that cannot be retrieved entirely from gross inspection and radiological analysis. In fact, some studies consider that the timing of fractures determined radiographically, especially in children, may be uncertain [67,68]. The determination of the posttraumatic survival interval is important not only to understand how past populations coped with injury but also because it assumes particular relevance in forensic contexts [47]. For most of the sample studied, however, it was difficult to evaluate the impact of factors such as age in the regular progression of the bone healing, a fact that may affect the estimation of the posttraumatic survival interval. Secondly, histology was useful to distinguish between calluses of fracture origin from other etiologies (e.g. subperiosteal hematoma, Sk. 119 individual). In this regard, it was also important to further corroborate evidence of secondary complications (e.g. osteomyelitis, Sk. 54 individual), showing that histology may complement the differential diagnosis. Thirdly, it revealed that a gap between the macroscopic and the histological morphology of the bone callus may exist. One can infer that the outer shell is consolidated, but not that the bone callus is completely healed or remodeled. Accordingly, researchers should be cautious when describing bone lesions of traumatic origin. Finally, histology was useful to determine the sequence of traumatic events in the Sk. 1,196 individual. Moreover, it revealed a set of microstructural changes compatible with age-related osteopenia and, eventually, osteoporosis. It is recommended that future studies should consider a similar approach when trauma lesions and the degree of bone healing are under the scope.
Acknowledgments
The authors would like to acknowledge Prof. Dr. Ana Luísa Santos and the Museu Bocage (Museu Nacional de História Natural) in Lisbon, in particular the former director, Dr. Maria da Graça Ramalhinho, and the former curator, Dr. Diana Carvalho for giving permission to collect the bone samples. This research was developed within a PhD project funded by the Fundação para a Ciência e Tecnologia (grants SFRH/BD/36739/2007 and UID/ANT/00283/2013).