Abstract
The circadian clock is rhythmically expressed in blood vessels, but the interaction between the circadian clock and disturbed blood flow remains unclear. We examined the relationships between BMAL1 and CLOCK and 2 regulators of endothelial function, AKT1 and endothelial nitric oxide synthase (eNOS), in vascular regions of altered blood flow. We found that the aortic arch from WT mice exhibited reduced sensitivity to acetylcholine (Ach)-mediated relaxation relative to the thoracic aorta. In Clock-mutant (mut) mice the aorta exhibited a reduced sensitivity to Ach. In WT mice, the phosphorylated forms of eNOS and AKT were decreased in the aortic arch, while BMAL1 and CLOCK expression followed a similar pattern of reduction in the arch. In conditions of surgically induced flow reduction, phosphorylated-eNOS (serine 1177) increased, as did p-AKT in the ipsilateral left common carotid artery (LC) of WT mice. Similarly, BMAL1 and CLOCK exhibited increased expression after 5 days in the remodeled LC. eNOS expression was increased at 8 p.m. versus 8 a.m. in WT mice, and this pattern was abolished in mut and Bmal1-KO mice. These data suggest that the circadian clock may be a biomechanical and temporal sensor that acts to coordinate timing, flow dynamics, and endothelial function.
Introduction
The force and composition of circulating blood is an important stimulus to the vasculature, affecting endothelial signals that are relayed to smooth muscle to cause functional responses of the blood vessel and ultimately affect blood flow. Intrinsic baseline differences in hemodynamics occur in the vascular tree due to the nature of circulatory system anatomy. Vascular bifurcations and curvatures such as the aortic arch are known to be regions of turbulent blood flow, whereas unbranched vessels exhibit laminar flow [1,2]. Changes in hemodynamics can also occur acutely in response to obstructions, exercise, and alterations in pressor and vasodilator mediators. These differences in blood flow have functional consequences. Turbulent blood flow predisposes the vasculature to atherosclerotic disease and laminar flow is generally atheroprotective [1,2]. The circadian clock has emerged as an important mechanism in key aspects of flow hemodynamics, including blood pressure [3,4,5,6,7], vascular tone [8,9], and vascular remodeling [10]. Mutation of Clock in cardiomyocytes in mice alters metabolic adaptation to increased fatty acid availability [11], and myofilaments lose time of day-dependent maximal calcium-dependent ATP consumption [12] while global Clock gene mutation in mice has detrimental effects in atherosclerosis [13] and vascular remodeling [8]. However, whether the circadian clock can respond to changes in hemodynamics that precede these pathological events is not known.
This circadian clock is a negative feedback loop that includes BMAL1, CLOCK, Period, and Cryptochrome. The heterodimer of CLOCK and BMAL1 are transcriptional activators that induce the Period and Cry genes, which then suppress the transcription of Bmal1 and Clock. Additional layers of molecular regulation confer the ability of the circadian clock to be a unique oscillating sensor, responding to environmental cues (zeitgebers). The day/light cycle is a zeitgeber that controls brain clock function, particularly in the suprachiasmatic nucleus or the central clock. Additionally, peripheral clocks respond to other cues. These zeitgebers have the capacity to change the phase of the oscillating clock or reset it each day, and in turn the circadian clock can then impact target genes. For example, in the liver, food is a known zeitgeber to control liver clock oscillations [14]. Alterations in the light cycle similarly alter central clock oscillation [15]. While peripheral clocks can act autonomously, there is also communication from the central clock to peripheral clocks [16]. Similar to the way in which the circadian clock is a sensor to cues from the environment, the vascular endothelium is also a sensor to the cues of flowing blood. The endothelium can respond via eNOS (endothelial nitric oxide synthase) and AKT to control vascular function. Furthermore, there is a connection between circadian rhythm and cardiovascular function in the heart rate, blood pressure, endothelial function, and fibrinolytic activity. However, whether fluctuations in vascular hemodynamics influence clock function is currently not known.
eNOS is an important regulator of every aspect of vascular function, including contractility [17], blood pressure [18,19], platelet aggregation [20], and vascular remodeling [21,22]. AKT, which is a critical regulator of eNOS, via phosphorylation of the serine 1177 residue, can profoundly promote nitric oxide production via this posttranslation modification [23,24]. Similarly, AKT is highly regulated by its threonine 308 residue by PDK. Interestingly, the circadian clock has been implicated in the regulation of both eNOS and AKT. Bmal1-KO and Per2 mutant mice exhibit endothelial dysfunction [8,9], which is accompanied by a decrease in eNOS and eNOS phosphorylation [25]. Similarly, both Bmal1-KO and Per2-KO mice have been demonstrated to have aberrant AKT, PI-3 kinase, and PDK expression [8,26]. With regard to hemodynamic regulation of eNOS, it has been shown to be stimulated by shear stress and circumferential stress, while AKT has also been demonstrated to be altered in response to changes in flow. In the studies reported here, we begin an investigation to determine if the circadian clock components BMAL1 and CLOCK have the capacity to respond to hemodynamic and remodeling forces, and examine the potential relationship with AKT and eNOS.
Materials and Methods
Animals
All animal studies were performed according to protocols approved by the Medical College of Georgia Institutional Committee for Use and Care of Laboratory Animals at Augusta University. Bmal1 and Clock heterozygote mutant mice, and Per2-Luc knockin mice were purchased from Jackson laboratories. All studies utilized double mutant mice or knockouts. mut mice were formerly generated by ENU mutagenesis in C57BL/6J mice causing an A to T transversion at the third base position of the 5′ splice donor site of intron 19 of Clock. All the mice were housed under standard 12-hour light/dark conditions.
Materials
p-eNOS (1177), eNOS, and p-AKT (threonine-308) monoclonal antibodies came from BD Transduction Labs. AKT polyclonal antibody came from Cell Signaling. BMAL1, CLOCK, and NPAS2 polyclonal antibodies came from Santa Cruz. GAPDH monoclonal antibody was from Ambion.
Functional Studies in Isolated Aorta and Aortic Arch
Fifteen- to 20-week-old Clock WT and mut male mice were anesthetized with ketamine/xylazine and subsequently exsanguinated. Residual blood was removed by perfusing physiologic saline by cardiac puncture over 3-5 min at physiologic pressures as previously described [22]. The thoracic aorta and aortic arch were carefully excised and placed into Krebs-Henseleit bicarbonate buffer solution. Adventitial fat was carefully removed and the arteries were cut into rings (2 mm thick). The rings were suspended by 2 tungsten wires (25 μm diameter) and mounted in a vessel myograph (6 mL chamber size; Multi Myograph, Danish Myo Technology). The isometric tension was measured using a force transducer coupled to a data acquisition system. A resting tension of 1.0 g was used throughout the experiments. After an equilibration period of 60 min (during which time KHS was changed every 10 min and the resting tension was readjusted), rings were precontracted with phenylephrine until a plateau was reached. Vessels were then washed with KHS and this was repeated at least 3 times in order to stabilize the tissue. Aortas and aortic arches were then precontracted with phenylephrine and concentration-dependent responses to the endothelium-dependent agonist, acetylcholine (Ach; 1 × 10-9 - 5 × 10-5M). Functional studies were performed at a single time point at noon.
External Carotid Artery Ligation
Mice were anesthetized with ketamine/xylazine. The distal left common carotid artery (LC) and its bifurcation into the external and internal carotid were exposed using minimal dissection. 8-0 nylon sutures (USSC Sutures, Norwalk, CT, USA) were then used to ligate the LC artery just proximal to the external/internal carotid artery bifurcation and the incisions were closed (5-0 suture). The left and right carotid artery were isolated for Western blotting 3, 4, and 5 days after ligation.
Western Blotting
Four individual aorta, aortic arch, ligated left carotid arteries, and right carotid arteries from the groups of WT mice were pooled to permit the detection of specific proteins, pulverized on dry ice, and then immersed into protein lysis buffer. Vessels were harvested in studies at noon, or at 8 a.m. versus 8 p.m. as indicated.
Period-2 Luciferase
Reporter mutant mice that express Period-2 Luciferase (PER2::LUC) were quantified with bioluminescence that reflects the endogenous Per2 expression. Briefly, LC undergoing ligation or nonligated controls were isolated from Per-2 luciferase knockin mice, homogenized, and then quantified by standard bioluminescence using automated luciferin dispensation. We have previously demonstrated kinetics and oscillation in the aorta using PER2::LUC knockin mice [10].
Immunohistochemistry
Aortic arteries and carotid arteries were dissected and rapidly embedded for frozen cross-sectioning. Sections were cut at 5 μm and mounted onto glass slides. BMAL1 and CLOCK were immunohistochemically detected. Briefly, the indirect avidin biotin-horseradish peroxidase visualization method was used (ABC Standard and Elite, Vector Red; Vector Laboratories, Burlingame, CA, USA). The primary antibodies utilized included Bmal polyclonal antibody (dilution 1:100; ABR Biotechnology, Abcam, Cambridge, MA, USA) and Clock polyclonal antibody (1:100; ABR Biotechnology, Abcam).
Statistics
Data are the mean ± SEM, with n referring to the number of mice per group. Significant differences were analyzed using a Student t test or ANOVA followed by a Bonferroni multiple-comparison post hoc test. Differences were considered statistically significant with p < 0.05.
Results
Endothelium-Dependent Relaxation to Ach in Aortic Arch and Aorta in WT and Clock Mutant Mice
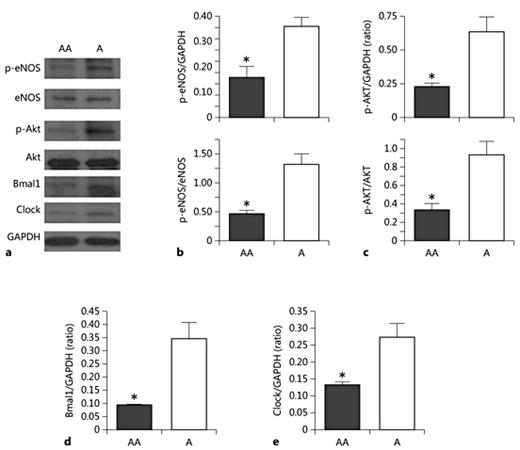
The aortic arch is a region of turbulent blood flow while the descending thoracic aorta exhibits laminar blood flow. To determine if vascular function was reduced in the aortic arch we undertook comparative studies between the 2 regions of vessels, and examined responses in WT and mut mice. Aortic arches from WT mice exhibited reduced sensitivity to Ach (1 nmol/L to 10 µmol/L) relative to WT descending thoracic aortas (Fig. 1a). The aorta from mut mice also exhibited reduced Ach sensitivity such that its relaxant response to Ach was indistinguishable from the aortic arch from mut (Fig. 1b). Comparison of the response curves of aortic segments from WT versus mut mice revealed that there was reduced sensitivity to Ach in mut mice (Fig. 1c). However, comparison of the aortic arches between WT and mut mice revealed no major difference in responsiveness to Ach, with mut arches tending to have a slightly improved response (Fig. 1d). When measured at a single time point (noon), the reduced response in endothelial relaxation to Ach of the aortic arch was reflected as a decrease in phosphorylated eNOS (Fig. 2a, b) and also AKT in WT mice (Fig. 2a, c). Interestingly, Bmal1 (Fig. 2d) and Clock expression (Fig. 2e) were also reduced in the aortic arch relative to the aorta. We then investigated the expression in thoracic aortas of WT, mut, and also Bmal1-KO mice at 2 time points, 8 a.m. and 8 p.m. eNOS expression exhibited a variation in expression in WT mice that was abolished in both mut (Fig. 3a) and Bmal1-KO mice (Fig. 3d). While neither Akt nor p-Akt exhibited a temporal variation in this 2-time point analysis (Fig. 3b, c, e), p-Akt expression was reduced in Bmal1-KO mice (Fig. 3f) as we have previously reported [8].
Endothelium-dependent relaxation of the aorta and aortic arch in WT and mutant mice. Endothelium-dependent relaxation to Ach in aortic arches and aortas of WT and mut mice are shown. a The responses in aortic arches were significantly reduced relative to the aortas of WT mice. b In the aorta of mut mice, impaired relaxant responses to Ach were observed, such that aortic function was not different from mut aortic arches and aortas. c The WT aorta from a versus the mut aorta from b. d The WT aortic arch from a versus the mut aortic arch from b. n = 5-8/group. * p < 0.05 at 10-8 to 10-5M Ach by 2-way ANOVA, Bonferroni post hoc test.
Endothelium-dependent relaxation of the aorta and aortic arch in WT and mutant mice. Endothelium-dependent relaxation to Ach in aortic arches and aortas of WT and mut mice are shown. a The responses in aortic arches were significantly reduced relative to the aortas of WT mice. b In the aorta of mut mice, impaired relaxant responses to Ach were observed, such that aortic function was not different from mut aortic arches and aortas. c The WT aorta from a versus the mut aorta from b. d The WT aortic arch from a versus the mut aortic arch from b. n = 5-8/group. * p < 0.05 at 10-8 to 10-5M Ach by 2-way ANOVA, Bonferroni post hoc test.
Reduced p-AKT, p-eNOS BMAL1, and CLOCK in the aortic arch relative to the aorta. The aorta or aortic arch was isolated from mice at 10 a.m., and protein lysates were made. Fifty micrograms of protein were run on SDS-PAGE electrophoretic gel and transferred to nitrocellulose membrane and probed with antibodies. a Representative Western blot of p-eNOS total eNOS, p-AKT, total AKT, BMAL1, CLOCK, and GAPDH. Densitometric quantification p-eNOS and total eNOS (b), p-AKT and total AKT (c), BMAL1 (d), and CLOCK (e). n = 4. * p < 0.05. AA, aortic arch; A, aorta.
Reduced p-AKT, p-eNOS BMAL1, and CLOCK in the aortic arch relative to the aorta. The aorta or aortic arch was isolated from mice at 10 a.m., and protein lysates were made. Fifty micrograms of protein were run on SDS-PAGE electrophoretic gel and transferred to nitrocellulose membrane and probed with antibodies. a Representative Western blot of p-eNOS total eNOS, p-AKT, total AKT, BMAL1, CLOCK, and GAPDH. Densitometric quantification p-eNOS and total eNOS (b), p-AKT and total AKT (c), BMAL1 (d), and CLOCK (e). n = 4. * p < 0.05. AA, aortic arch; A, aorta.
Time-of-day-dependent variation in eNOS in WT mice is abolished in mut and Bmal1-KO mice. Protein lysates extracted from the aorta at either 8 a.m. or 8 p.m. were used for Western blotting and quantified by densitometry for eNOS (a), Akt (b), and p-Akt (c) in WT and mut mice, and also in Bmal1-KO mice (d-f). An increase in eNOS expression was observed in WT mice at 8 p.m. versus 8 a.m. in WT mice, an effect that was abolished in mut (a) and Bmal1-KO mice (b). While neither Akt nor p-Akt oscillated in WT mice (b, c, e), p-Akt expression was reduced in Bmal-KO mice (f). * p < 0.05 versus the corresponding WT time point, +p < 0.05 mut versus WT at the same time point.
Time-of-day-dependent variation in eNOS in WT mice is abolished in mut and Bmal1-KO mice. Protein lysates extracted from the aorta at either 8 a.m. or 8 p.m. were used for Western blotting and quantified by densitometry for eNOS (a), Akt (b), and p-Akt (c) in WT and mut mice, and also in Bmal1-KO mice (d-f). An increase in eNOS expression was observed in WT mice at 8 p.m. versus 8 a.m. in WT mice, an effect that was abolished in mut (a) and Bmal1-KO mice (b). While neither Akt nor p-Akt oscillated in WT mice (b, c, e), p-Akt expression was reduced in Bmal-KO mice (f). * p < 0.05 versus the corresponding WT time point, +p < 0.05 mut versus WT at the same time point.
We next examined the response to arterial ligation in WT mice. We and others have shown that arterial ligation alters flow and shear stress, in particular during the ligation of the common carotid artery [27]. We implemented the external carotid artery ligation model of flow reduction, which occludes blood flow through from the common carotid artery to the external carotid artery, diverting blood flow only through the internal common carotid branch, which causes a reduction in blood flow in the common carotid artery. We assessed changes in protein expression 3, 4, and 5 days after this ligation, which represent time points that transition the vasculature from a toned vessel to remodeled vessel. After 3 days after ligation, p-eNOS, total eNOS, p-AKT, and total AKT expression were very similar between the unligated control right common carotid artery (RC) and LC. At 4 days, while total eNOS and total AKT were not different between RC and LC expression (Fig. 4a), it appeared that both p-eNOS and p-AKT expression had increased (not significantly) in both the RC and LC relative to 3 days. By day 5 after ligation, p-eNOS (Fig. 4b, top) increased significantly in LC as did p-AKT (Fig. 4c, left). When normalized to total eNOS, p-eNOS remained elevated and significant in LC (Fig. 4b, bottom). However, an increase in total AKT on day 5 may have also partially accounted for the increase in p-AKT (Fig. 4c, right). Interestingly, BMAL1 and CLOCK exhibited no changes in expression on day 3 and day 4 postligation, but did exhibit an increase in both BMAL1 and CLOCK expression on day 5 in ligated LC (Fig. 4d, e). We then conducted ligation studies in the reporter Per2-Luciferase/Luc knockin mice, which reveal Per2 oscillation similar to endogenous Per2 expression. These mice have an intact and functional circadian clock (including Per2) but serve as a reporter for the circadian clock using the Per2 tagged with luciferase. Per2-luc activity was increased in ligated LCs (Fig. 4f), which is consistent with the upregulation of Bmal1 and Clock proteins (Fig. 4d, e) as Bmal1 and Clock drive Per2 transcription. These changes in BMAL1 and CLOCK observed in the aortic arch and ligated common carotid artery were distributed across the vasculature evident both in the endothelium and smooth muscle, while staining was absent in Bmal1-KO mice (Fig. 5).
Increased p-AKT, p-eNOS, BMAL1, and CLOCK after external carotid artery ligation in WT mice. WT mice underwent left external carotid artery ligation for 3, 4, and 5 days to cause a flow reduction in the LC. LC and contralateral control RC were then isolated and processed for immunoblotting. The representative Western blot (a) and densitometry show a significant increase in p-eNOS (b), p-AKT (c), BMAL1 (d), and CLOCK (e) in the LC after 5 days of ligation compared to the RC. f Per2 promoter activity in Per2 knockin mice postligation. Per2-Luc knockin mice underwent ligation for 2 weeks, and common carotid arteries were harvested and pulverized, and subsequently analyzed for bioluminescence after the addition of luminol substrate. n = 4. * p < 0.05.
Increased p-AKT, p-eNOS, BMAL1, and CLOCK after external carotid artery ligation in WT mice. WT mice underwent left external carotid artery ligation for 3, 4, and 5 days to cause a flow reduction in the LC. LC and contralateral control RC were then isolated and processed for immunoblotting. The representative Western blot (a) and densitometry show a significant increase in p-eNOS (b), p-AKT (c), BMAL1 (d), and CLOCK (e) in the LC after 5 days of ligation compared to the RC. f Per2 promoter activity in Per2 knockin mice postligation. Per2-Luc knockin mice underwent ligation for 2 weeks, and common carotid arteries were harvested and pulverized, and subsequently analyzed for bioluminescence after the addition of luminol substrate. n = 4. * p < 0.05.
Immunohistochemistry of BMAL1 and CLOCK staining in aortic arteries and common carotid arteries. Immunohistochemical detection of BMAL1 and CLOCK protein expression (brown) decreased in aortic arches and increased in ligated LC, suggesting that BMAL1 and CLOCK were capable of detecting flow alterations, counterstained with hematoxylin (blue). The final image is a Bmal1-KO carotid artery immunostained with Bmal1, demonstrating limited staining. Colors refer to the online version only.
Immunohistochemistry of BMAL1 and CLOCK staining in aortic arteries and common carotid arteries. Immunohistochemical detection of BMAL1 and CLOCK protein expression (brown) decreased in aortic arches and increased in ligated LC, suggesting that BMAL1 and CLOCK were capable of detecting flow alterations, counterstained with hematoxylin (blue). The final image is a Bmal1-KO carotid artery immunostained with Bmal1, demonstrating limited staining. Colors refer to the online version only.
Discussion
The circadian clock has a profound influence in the cardiovascular system. Blood pressure [28,29,30], vascular remodeling [8,10,31,32,33], atherosclerosis [13], cardiac injury [34,35,36,37], and coagulation [38] are all influenced by circadian clock genes. Thus, this molecular regulator of 24-h rhythmicity controls not only daily and acute pathophysiology, such as blood pressure and thrombosis, but also chronic responses. The circadian clock serves to control physiology by anticipating through timing, which is related to sensing internal environmental stimuli that change with time. This also includes an ability to respond to changes that occur with disease and aging [39,40]. An intriguing possibility is that the circadian clock might also be a sensor to changes in hemodynamics and remodeling in the cardiovascular system. While BMAL1 and CLOCK are nuclear transcription factors, The BMAL1-CLOCK heterodimer has been demonstrated to shuttle from the cytoplasm to the nucleus via nuclear localization and export signals in the PAS domain of BMAL1 [41], which may permit responsiveness to even blood-borne signals. In the present study, we examined the influence of altered flow dynamics on vascular function, signaling, and clock function. Within the vasculature tree, the aortic arch is prone to atherosclerosis in part due to its physical curvature and its branch points, which include the common carotid arteries and subclavian arteries [2,42,43]. In these regions, blood flow is turbulent and thus more susceptible to atherosclerosis and endothelial dysfunction [1]. We did find reduced endothelial function in the aortic arches of WT mice as measured by the relaxant response to Ach, which could cause susceptibility to atherosclerosis during hypercholesterolemic conditions. In the Watanabe heritable hyperlipidemic rabbits, relaxant responses of the aortic arch to Ach were also shown to be smaller than the response in the aorta [42]. In the apolipoprotein E (apoE) knockout mouse that exhibits hypercholesterolemia and atherosclerosis, calcium signaling has been shown to be greater in the aortic arch than in the aorta [43]. In the mut mouse, we found that the aorta exhibited reduced relaxant responses to Ach such that the response resembled that of the aortic arch of mice of either genotype. The reduction in Ach-mediated relaxation in mut mice is consistent with previous observations and other circadian mut mice, including Bmal1-KO and Per2-KO mice [8,9]. The impaired relaxation to Ach may also contribute to the increased susceptibility to atherosclerosis in circadian mut mice, as has been shown in mut/apoE double knockout mice [13] In addition, global Bmal1-KO mice crossed to LDL receptor-KO mice exhibit increased atherosclerosis when fed a high-fat diet [44]. Conversely, an atheroprotective phenotype is observed when the Cryptochrome gene is overexpressed locally by adenovirus in apoE knockout mice [45].
AKT1 and eNOS play pivotal roles in the regulation of endothelial function and both respond to shear forces [23,24,46,47,48]. We found that AKT and eNOS expression were reduced in the aortic arch relative to the aorta. BMAL1 and CLOCK expression were also reduced in the aortic arches of WT mice. With arterial ligation in the common carotid artery, total eNOS did not change in response, which is consistent with the observation that endothelial function is not affected in this model [27]. However, p-eNOS and p-AKT did increase after 5 days of ligation, as did BMAL1 and CLOCK. Thus, circadian clock expression was altered in response to acute changes in blood flow and remodeling. These changes in expression of BMAL1, CLOCK, AKT, and eNOS after ligation may thus be the consequence of remodeling and altered hemodynamics.
In conclusion, we demonstrated differential expression patterns of BMAL1 and CLOCK that mirror changes in p-AKT and p-eNOS in conditions of chronically altered blood flow. The ability of the circadian clock to respond to flow and remodeling changes may suggest an important role not only as a temporal anticipator, but also as a function as a blood flow sensor in the vasculature.
Disclosure Statement
The authors have no disclosures.