Abstract
Over 50% of the human body is comprised of fluids that are distributed in defined compartments. Although compartmentalized, these fluids are dynamically connected. Fluids, electrolytes, and acid-base balance in each compartment are tightly regulated, mostly in an energy-dependent manner to achieve their designed functions. For over a century, our understanding of the microvascular fluid homeostasis has evolved from hypothesized Ernest Starling principle to evidence-based and the revised Starling principle, incorporating the functional endothelial surface layer. The kidney is a highly vascular and encapsulated organ that is exquisitely sensitive to inadequate (insufficient or excess) blood flow. The kidney is particularly sensitive to venous congestion, and studies show that reduced venous return triggers a greater degree of kidney damage than that from lacking arterial flow. Thus, fluid overload can induce severe and sustained kidney injury. In the setting of established acute kidney injury, fluid management can be challenging. Impaired capacity of urine output and urine concentration and dilution should be taken into consideration when designing fluid therapy. Video Journal Club ‘Cappuccino with Claudio Ronco' at http://www.karger.com/?DOI=452702.
Introduction
Fluid homeostasis in humans is tightly regulated under physiological conditions. Alterations of fluid volume and electrolyte balances can have multiple deleterious effects. In this review, we integrate old and new knowledge with in-depth evaluation of the effects of fluid therapy on renal hemodynamics and potential injury to the kidney.
Fluid Physiology
The human body is comprised of approximately 60% water, which is distributed one-third (20%) to extracellular and two-thirds (40%) to intracellular spaces. The extracellular fluids (ECF) are further distributed to interstitial and intravascular spaces, 80 and 20%, respectively. Thus, a 70 kg adult is comprised of ∼42 kg (liters) body water, 28 liters intracellular fluids and 14 liters ECF. The latter is further distributed to 11 liters interstitial fluids and ∼3 liters plasma. Electrolyte and acid-base regulations in each compartment are highly regulated, mostly in an energy-dependent manner, and have a narrow physiological window. Under physiological conditions, albumin leaks out of circulation through capillary pores at a rate of approximately 5% per hour and returns to the circulation via the lymphatic system and the thoracic duct [1]. Extensive and dynamic exchanges exist between the ECF and gastrointestinal system as well as the kidneys. There is a turnover of 8-9 liters/day of gastrointestinal fluids (secretion and absorption) [2] and 180 liters/day of glomerular filtrates of which 98-99% is absorbed by renal tubules. Multiple factors, including a large number of medications, could affect these balances.
Vascular Fluid Homeostasis
Tissue health is dependent on adequate oxygenation. Tissue oxygenation is the main function of the microcirculation. Over the years, the concept of microcirculation originated by Ernest Starling (classic Starling principle) in 1896 [3] has been challenged by a number of new discoveries, and a revised Starling principle emerged [4].
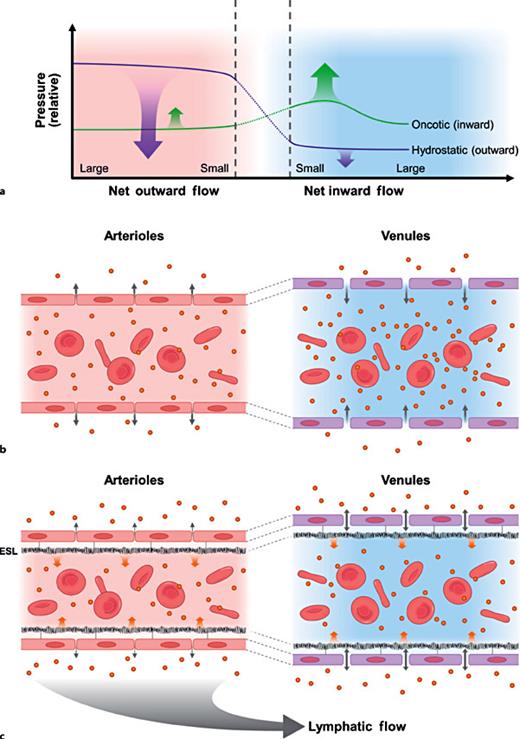
The classic Starling principle (1896) proposes that transport of fluids to and from the interstitial space of peripheral tissues is balanced between opposing oncotic and hydrostatic forces (pressure) across the vessel wall (fig. 1a, b) [3]. Emerging evidence does not support this concept. The classic principle was called into question by the discovery, both by imaging and by functional studies [5,6,7,8], of a negatively charged, gel-like glycocalyx layer (when binding with plasma constituents, it is also known as the endothelial surface layer, ESL), covering the surface of the endothelium (fig. 1c). ESL was shown to be present throughout the vasculature but was more prominent in vessels with a higher magnitude of hydrostatic pressure [9]. The first recognition of substantial contribution of the endothelial glycocalyx, the ESL, to the vascular barrier dates back to the early 1980s [10]. Levick famously noted ‘low lymph flow paradox', in that vascular barrier remains competent even when the interstitial oncotic pressure equals that in vascular lumen [11,12]. Studies have also demonstrated that the oncotic gradient between the vessel lumen and interstitium is, in fact, minimal and would not account for any gradient-driven inward fluid shift [13,14]. Movement of fluids into the interstitial space is largely prevented by the opposing oncotic and hydrostatic pressures within vascular lumen at the level of ESL, not across the vessel wall [12]. It has become clear that the ESL plays a pivotal role in preventing excessive vascular fluids extravasation, thus preventing the formation of tissue edema. Indeed, there has been no evidence showing that interstitial fluids can be reabsorbed into the venular site of the vessel through oncotic pressure gradient [15]. More than a major regulator of the plasma fluid filtration [4,16], the ESL has proven to be important in providing vascular protection [17] and regulation of microvascular blood flow [18,19]. It mediates sheer stress from the blood flow through the endothelial cells [20] and prevents leukocyte-endothelium and platelet-endothelial adhesion [21].
The evolution of the Starling principle of fluid dynamics. a The classic Starling principle (1896), hydrostatic and oncotic pressures across microvascular wall. The net sum of hydrostatic outward pressure and oncotic inward pressure causes a net fluid outflow from the vessel at the arteriole side and a net inward fluid flow in the venular side. b Schematic illustration of the classic Starling principle. Fluids are filtered outward into the interstitium and returns to the circulation via post capillary venules. Note: it was assumed that in the post capillary venules, the oncotic pressure was higher than that in the interstitial space. The assumption has since been disproved. c The revised Starling principle incorporating the known evidence emerged in the last half century.
The evolution of the Starling principle of fluid dynamics. a The classic Starling principle (1896), hydrostatic and oncotic pressures across microvascular wall. The net sum of hydrostatic outward pressure and oncotic inward pressure causes a net fluid outflow from the vessel at the arteriole side and a net inward fluid flow in the venular side. b Schematic illustration of the classic Starling principle. Fluids are filtered outward into the interstitium and returns to the circulation via post capillary venules. Note: it was assumed that in the post capillary venules, the oncotic pressure was higher than that in the interstitial space. The assumption has since been disproved. c The revised Starling principle incorporating the known evidence emerged in the last half century.
The endothelial glycocalyx consists primarily of membrane bond glycoproteins (adhesion molecules, selectin and integrins) and proteoglycans (syndecans and glypicans and side chains containing heparin and chondroitin sulphates) [7]. ESL has a functional thickness of about 1 µm (reviewed in [6,19]). General consensus is that its width varies in different regions of vasculature. Large vessels show thicker/wider ESL than those in microvessels [9]. The relative contributions of the major GAGs to the thickness of the ESL and to its small solute permeability have been demonstrated in rodent mesenteric vessels [22]. There is a dynamic equilibrium between the proteins in the blood stream and those bound to the ESL [6,23]. A small space of <100 nm beneath the ESL is almost protein free, and this generates an oncotic pressure gradient to deter the passage of protein outward (inwardly directed oncotic gradient) within the vessel lumen [13]. The ESL also lowers hydraulic conductivity (fluid filtration rate/area/pressure gradient), thus retarding hydrostatic pressure-mediated fluids extravasation [24].
The ESL can be compromised during ischemia-reperfusion injury, sepsis, inflammation, hypervolemia, diabetes and oxidized lipids [25,26,27,28,29,30,31]. Inflammation increases the permeability of the endothelial glycocalyx layer to polyionic macromolecules allowing fluids to move into the interstitium [32,33,34]. Volume expansion triggers the production of atrial natriuretic peptides (ANP) from the atrium. ANP is capable of effectively degrading endothelial glycocalx, leading to ESL injury and barrier failure [25,35].
Fluid Requirement in Healthy and in Hospitalized Patients
Maintenance requirement for water in adults under physiological condition is 25-35 ml/kg/day. Daily requirements of sodium (Na) and potassium (K) are approximately 1 mmol/kg/day. The kidneys are endowed with an impressive capacity to conserve Na. They are, however, woefully unprepared to deal with the opposite, Na excess. Thus, humans cope with low Na intake much better than high Na intake. Such a trait is hereditary and is a result of millions of years of terrestrial evolution in which humans, in response to the Paleolithic (low salt) diet [36], have adapted physiologically and metabolically to retain Na. It is not surprising that overabundance of Na intake, which has occurred in the last decades, has been linked to the genesis of a number of serious and chronic diseases, including hypertension and cardiovascular diseases [37,38]. Similarly, overuse of crystalloid infusion in hospital has resulted in multiple complications including mortality, and this finding has been summarized and confirmed in a recent study [39].
Fluid administration is the most common intervention prescribed to hospitalized patients. Improper fluid prescription can cause multiple adverse effects and contribute to patients' mortality [40]. Whether or not to administer the intravenous fluids should be based on clinical settings. If the patient is hemodynamically stable and is made NPO for <8 h for an elective procedure, no maintenance fluids are required [41]. In patients post-abdominal open surgery, even fluid balance postoperatively over the course of up to 5 postoperative days is important to a better clinical outcome compared to those with fluid imbalance, mostly with a net positive balance [40].
For patients with complicated cardiovascular surgery such as open repair of abdominal aortic aneurysms and cardiac valve replacement, postoperative vasoplegia is common, largely related to extensive tissue injury and damage-dependent molecular patterns [42]. Treatment requires a combination of isotonic fluids and vasoactive agents. For patients with sepsis and septic shock, fluids in combination with pressors are required especially for patients during the initial resuscitation period [43].
If the IV fluids are deemed necessary, crystalloid fluids are, in general, preferred to colloid fluids with a few exceptions. In patients with acute hemorrhagic shock, blood products are preferred to maintain volume status and tissue perfusion. In patients with cirrhosis and advanced hepatic failure, albumin-containing fluids and, recently, albumin dialysis can be beneficial [44]. There are conditions under which colloids could be detrimental. For instance, in patients with acute brain trauma, albumin-containing fluids have been shown to increase complications, including mortality [45,46]. Semi-synthetic colloid solutions, such as hetastarch-containing fluids, are no longer advised clinically because of multiple adverse effects, including (1) increased occurrence of kidney dysfunction, (2) greater need for blood transfusion, and (3) increased mortality in certain clinical settings [47,48]. The detrimental effects of semi-synthetic colloids may have been related to the accumulation of low molecular weight fraction in proximal renal tubules and osmotic nephrosis [49].
Among crystalloid fluids, balanced fluids are preferred to high chloride-containing fluids, that is, 0.9% saline. In balanced crystalloid fluids, metabolizable anions are incorporated to substitute bicarbonate and the final components of a balanced crystalloid resemble the constituents of normal plasma [50]. High chloride-containing saline, that is, 0.9% saline contains super physiological content of chloride which, when infused in large quantities (>2 liters), can cause hyperchloremic acidosis, which may engender complications including coagulopathy, hyperkalemia and more pronounced interstitial fluid retention; 0.9% saline infusion also causes greater fluid retention (∼60% by 6 h post infusion) than that of 5% dextrose in normal adults [51]. Moreover, Chowdhury et al. [52] have shown that 56% of infused saline was retained in the body 6 h after the infusion in healthy volunteers, compared to 30% retention of Hartmann's solution [53]. When 0.9% saline was compared to plasmalyte, calculated interstitial volume increased more with saline than with plasmalyte. Negative microcirculatory effects are exerted on the kidneys by 0.9% saline (detailed below).
In a few clinical scenarios, 0.9% saline is indicated. For patients with acute central nervous system events, 0.9% saline may be preferred, as it has an osmolality of 308 msOm/l, a slightly higher tonicity than that in the commonly used balanced fluids (i.e., lactated ringers). For patients with metabolic alkalosis due to upper GI diseases, gastric suction or chronic vomiting, 0.9% saline is an appropriate fluid of choice.
The goal of fluid resuscitation is to optimize hemodynamics, increase stroke volume, and ultimately improve organ perfusion and O2 delivery. In critically ill patients, the Sepsis Campaign has advocated initial fluid resuscitation (up to 30 ml/kg) within the first 6 h of presentation [43]. After the initial resuscitation, persistent net positive fluid balance is not advised in general, as it can lead to interstitial fluid overload. Fluid overload and cumulative positive fluid balance have been shown to cause multiple adverse effects in multiple organ systems and increase mortality [54,55,56] and should be avoided. Current measurements for fluid status are indirect and mostly less than ideal, although a recent study in critically ill patients using bioimpedance has shown initial exciting results [39]. In recent years, several direct microcirculation monitoring techniques have also been devised [57]. Although initial studies have shown some correlation between mortality and the direct microcirculation measurement in early ICU admissions (≤48 h) [58,59,60], the results have not been consistent, especially in heterogeneous ICU patients [61]. Moreover, in their current state, the methods require cumbersome offline data analysis, not yet feasible for routine bedside use. These methods, nonetheless, provide hope for a better determination of the patient's microcirculatory status and tissue oxygenation to guide fluid therapy in the near future.
Kidneys are Sensitive to Fluids and Volume Status
The kidneys are sensitive to both arterial and venous flow. Insufficient arterial flow and or venous congestion can rapidly impair renal clearance. As the kidney is encapsulated and has little room to expand, venous congestion engorges the kidney, causing intracapsular pressure buildup, reducing both venous and lymphatic outflow as well as arterial blood inflow (fig. 2). Studies have shown that 30 min of ischemia by clamping the renal vein resulted in several fold higher of serum creatinine elevation than clamping of renal arteries for the same duration [62,63]. Even in a normal individual, kidney volume increases in response to crystalloid fluid infusion [52], indicating intra-renal fluid retention. Given the microvascular leakiness in critically ill patients, with fluid therapy, the intra-renal fluids retention and edema could be more prominent. The interstitial edema would impede forward arterial blood flow as well as venous return and lymphatic drainage, leading to an impaired tissue oxygenation, acute kidney injury (AKI) and further worsening congestion, creating a vicious cycle (fig. 2). Studies have shown that in patients with sepsis and after abdominal surgery, cumulative fluid balance is strongly associated with AKI occurrence and progression [64,65]. Patients who received conservative fluid administration and vasopressors tend to have favorable kidney outcome and decreased odds for the development of AKI [65]. Exogenous pressure to the kidneys due to obesity and abdominal ascites can also compromise renal blood flow both in the arterioles and the venues causing anuric AKI [66], analogous to the compartment syndrome.
Excess fluids and AKI. Schematic illustration of fluid infusion, when exceeding the capacity of lymphatic drainage in the microcirculation, will inevitably cause interstitial edema. In the kidney, interstitial edema increases subcapsular and intra-capsular pressure, leading to the reduction in forward renal arterial blood flow, reduction in venous return and lymphatic drainage, ultimately causing tissue hypoxia and AKI, analogous to compartment syndrome. Endothelial and ESL damage can also cause inflammation and microthrombosis, leading to sustained kidney injury. Inadequate urine output in AKI can further worsen tissue edema, creating a vicious cycle.
Excess fluids and AKI. Schematic illustration of fluid infusion, when exceeding the capacity of lymphatic drainage in the microcirculation, will inevitably cause interstitial edema. In the kidney, interstitial edema increases subcapsular and intra-capsular pressure, leading to the reduction in forward renal arterial blood flow, reduction in venous return and lymphatic drainage, ultimately causing tissue hypoxia and AKI, analogous to compartment syndrome. Endothelial and ESL damage can also cause inflammation and microthrombosis, leading to sustained kidney injury. Inadequate urine output in AKI can further worsen tissue edema, creating a vicious cycle.
Although 0.9% saline and balanced fluids can both lead to renal volume expansion, interstitial fluid retention and adverse intra-renal microvascular effects are more pronounced with 0.9% saline infusion [52,53]. Chowdhury et al. [52] clearly show that 0.9% saline infusion (2 liters), when compared to the same amount of balanced fluid infusion, causes more significant reduction in the renal cortical blood flow and oxygen delivery. Such differences likely are related, at least in part, to the super physiological chloride content in 0.9% saline, which activates the tubuloglomerular feedback among other renal tubular effects [67], causing afferent arteriole vasoconstriction [50]. Simultaneous venous congestion and afferent arteriole constriction further diminish forward blood flow [68]. In line with these mechanisms, studies have shown that postoperative hyperchloremia in patients with normal preoperative serum chloride is associated with postoperative renal dysfunction [69].
Chloride restrictive fluids have been associated with a lower hospital mortality and decreased incidence of AKI or need for renal replacement therapy in critically ill patients [70,71]. Recently, a multi-center prospective study evaluating 0.9% saline vs. balanced fluids in ICU patients, however, has shown no difference in the outcome [72]. The study, however, was focused on ICU patients, and the types of IV fluids given to the study participants prior to the ICU admission were unclear. In practice, critically ill patients typically receive large amounts of fluids during transit, in the ED, and prior to the ICU admission. Thus, further IV fluids in the ICU may not be sensitive enough to detect a difference. Also, large amounts of total fluids administration, regardless of the type of fluids, can be detrimental. Further studies are needed preferably to evaluate the total amount of fluid therapy in critically ill patients with a specific time frame from their initial presentation.
The rate of IV fluids is also likely to impact kidney function. Rapid IV infusion can transiently raise atrial pressure, resulting in ANP release. The latter rapidly degrades the endothelial surface glycocalax layer [27,30], compromising the barrier function. Intravenous fluids can readily enter the interstitial compartment, leading to intra-renal pressure build-up and AKI. Moreover, ESL damage can also cause inflammation and microthrombosis [6,8], leading to sustained kidney dysfunction.
For patients who have developed AKI and are in need of fluids, the current recommendations put forth by KDIGO (kidney disease improvement global outcome) are to use isotonic crystalloids in the absence of hemorrhage [73]. AKI predisposes to fluid infusion-associated alterations in electrolytes, acid-base and in tonicity as the kidney's concentration and diluting capacities are vastly compromised. Intravenous fluids should be used with caution to avoid volume overload and interstitial congestion (Box 1). Patients with new onset proteinuria and active urine sediment (renal tubular epithelial cells) but who fail to meet the conventional sCr criteria of AKI (Box 2) should be managed carefully to optimize perfusion pressure, volume status [64] and avoidance of situations or agents that could be injurious to the kidney.


Unfortunately, in practice, providers are inclined to use albumin infusion in patients with overt fluid overload such as dependent edema and pulmonary congestion in an attempt to ‘recruit interstitial fluids'. The endothelial barrier failure readily leaks infused albumin into the interstitium, resulting in an increase in the interstitial oncotic pressure and worsening interstitial edema and further diminishing O2 in the kidneys. It is not surprising that to date, no study has shown any outcome advantage of albumin infusion over crystalloid infusion for (non-cirrhotic) critically ill patients [74,75].
Taken together, kidneys consume large amounts of oxygen. The PO2 in the outer medullar is at ∼5-10 mm Hg under normal conditions. The kidney is thus sensitive to both insufficient and excessive fluid administration. Considering Fick's law, tissue O2 diffusion is determined by (1) the O2 gradient between capillary and tissue, (2) diffusional distance, and (3) area available for gas exchange [76], reduced renal arterial flow, interstitial edema, elevated intra-capsular pressure, and renal venous congestion can work in concert to deplete intra-renal O2 and incite inflammatory reaction, leading to persistent kidney injury.
Conclusion
Much progress has been made over the last several decades in our understanding of vascular homeostasis. Solid evidence indicates the existence of ESL and its functional significance in microcirculatory fluid homeostasis and vascular protection, including its role as a barrier of plasma fluids extravasation, sheer stress and receptor-mediated regulations, anti-thrombosis and anti-inflammation. The recognition of factors that can injure and compromise the ESL should caution us to be mindful in practice. Fluid management can impact ESL integrity, microcirculation and tissue O2 supply. In the kidney, suboptimal fluid therapy, insufficient or excessive, can cause AKI and worsen existing AKI. Uncertainty and controversy remain regarding appropriate fluid management for critically ill patients including AKI patients, mainly due to the lack of reliable methods to determine patient fluid status. Recently, a new generation of hand-held microscopy with automatic analysis software has generated excitement in the hope of providing a means to access vital organ microcirculatory status at the bedside in vivo and noninvasively to better guide fluid therapy in the near future. Due to the nonavailability of reliable volume assessment tools, clinicians continue to use a variety of surrogate markers for volume determination. Emphasis should nonetheless be placed on appropriate fluid selection and judicious fluid administration to avoid alterations in volume status and electrolyte balance, especially to avoid overt volume overload, which is a known cause of AKI, multiorgan failure and mortality. Fluid management for patients with existing AKI can be challenging. The impaired capacity to produce, concentrate and dilute urine can cause severe alterations in fluid and acid-base/electrolyte balance and should be taken into consideration when prescribing fluids for AKI patients.
Key Messages
• Fluid therapy should be prescribed based on the patients' clinical setting and daily requirements with a goal to restore patients to their physiological state.
• During initial resuscitation, treatment goals should include both hemodynamic stabilization and microcirculation optimization in order to maintain adequate tissue O2 supply.
• Fluid selection can impact patient outcome; appropriate fluid should be selected to avoid alterations in serum tonicity, electrolytes and acid-base balance.
• Preservation of the glycocalyx layer should be taken into consideration when prescribing fluids.
• Interstitial volume overload is associated with AKI, multiple complications and increased mortality.
• Kidney tolerates balanced fluids better than high-chloride fluids (i.e., 0.9% saline).
• Volume overload (regardless of fluid type) raises kidney intra-capsular pressure, causing AKI, analogous to the compartment syndrome.
• Newer volume-assessment methods and direct bedside determination of tissue microcirculation hold the future for a better determination of volume status to guild fluid therapy for critically ill and AKI patients.
Disclosure Statement
The authors have no competing interests.
Funding
None.