Abstract
Acute lung injury and acute respiratory distress syndrome (ARDS) represent a heterogenous group of lung disease in critically ill patients that continues to have high mortality. Despite the increased understanding of the molecular pathogenesis of ARDS, specific targeted treatments for ARDS have yet to be developed. ARDS represents an unmet medical need with an urgency to develop effective pharmacotherapies. Multiple promising targets have been identified that could lead to the development of potential therapies for ARDS; however, they have been limited because of difficulty with the mode of delivery, especially in critically ill patients. Nanobiotechnology is the basis of innovative techniques to deliver drugs targeted to the site of inflamed organs, such as the lungs. Nanoscale drug delivery systems have the ability to improve the pharmacokinetics and pharmacodynamics of agents, allowing an increase in the biodistribution of therapeutic agents to target organs and resulting in improved efficacy with reduction in drug toxicity. Although attractive, delivering nanomedicine to lungs can be challenging as it requires sophisticated systems. Here we review the potential of novel nanomedicine approaches that may prove to be therapeutically beneficial for the treatment of this devastating condition.
Introduction
Despite recent advances in diagnostic and therapeutic modalities, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) still represent an unmet medical need because of the high morbidity and mortality with substantial medical expenditure [1,2,3]. Hence, there is an urgent need to develop novel approaches to treat ARDS. Unfortunately, contemporary drug development approaches to address this challenge have not been rewarding - in particular blockade of single cytokines and chemokines have failed to show an improvement in outcomes because of the complex pathogenesis and nature of the disease [4]. Therefore, defining the contribution of proximal signaling pathways that amplify the inflammatory response and developing targeted therapies to specifically block them is an attractive approach to limit injury and inflammation for this devastating disease [5]. To this end, we have identified several targets that modulate signaling pathways that amplify inflammation and hence are attractive to be tested as potential therapies for ALI and ARDS. However, delivery of peptides, proteins, and silencing RNAs to the lung is an ongoing challenge [6,7,8].
Nanoscience is the study of nanoscale materials, processes, and devices. In recent years nanomedicine has become an attractive concept for the targeted delivery of therapeutic and diagnostic compounds to the lung [9,10]. Nanoparticle drug delivery systems can be used to provide delivery of drugs, improve bioavailability, and sustain the release of drugs for systemic delivery. These systems have the ability to improve the pharmacokinetics and pharmacodynamics of agents, allowing an increase in the biodistribution of therapeutic agents to target organs and resulting in improved efficacy while minimizing drug toxicity [11,12,13]. Nanocarriers are specially designed to target inflammation and cancer that have a permeable vasculature. Among the various drug delivery systems considered for pulmonary application, the use of biodegradable polymeric nanoparticles represents several advantages for the treatment of respiratory diseases [14,15]. These methods are particularly attractive for the delivery of molecular targets in the setting of critical illnesses such as adult respiratory distress syndrome and sepsis [7,8]. A number of different strategies have been proposed for modification of nanoparticle characteristics to control their behavior within biological environments, such as cell-specific targeted drug delivery or modified biological distribution of drugs, both at the cellular and organ levels. This method of delivery is particularly attractive for inflammatory conditions such as ALI and ARDS.
Accordingly, we have harnessed unique attributes of 3 novel, long-acting, biocompatible, and biodegradable anti-inflammatory nanomedicines and tested them in cell and murine models of inflammation. They consist of 2 amphipathic peptide drugs, human glucagon-like peptide-1(7-36) amide (GLP-1), triggering receptor expressed on myeloid cells (TREM1) blocking peptide, and 17-allylamino-17-demethoxygeldanamycin (17-AAG), a water-insoluble cytotoxic drug [5,16,17,18]. This innovative approach consists of self-assembly of each drug with US FDA-generally regarded-as-safe distearoylphosphatidylethanolamine covalently linked to polyethylene glycol of molecular weight 2,000 (DSPE-PEG2000), a component of US FDA-approved Doxil®, that forms long-acting, biocompatible and biodegradable, sterically stabilized phospholipid micelles in an aqueous milieu (SSM; size: ∼15 nm) [5,17,18,19,20].
Acute Lung Injury/Acute Respiratory Distress Syndrome
ALI and ARDS arise from direct and indirect injury to the lungs and result in a life-threatening form of respiratory failure with diffuse bilateral lung injury and severe hypoxemia caused by noncardiogenic pulmonary edema, which affects approximately 1 million people worldwide annually [1,3,21]. The importance of lung injury has been highlighted by the emergence of SARS (severe acute respiratory syndrome) and MERS (Middle Eastern respiratory syndrome) [21]. The major reason underlying the lag in improvement in outcome is the lack of novel and specific therapies for ALI and ARDS [2]. As with inflammatory processes, lung inflammation is accompanied by many cellular and biochemical processes, and injury to both the pulmonary capillary endothelium and the alveolar epithelium. Despite improved molecular understanding, development of specific treatments for ARDS have yet to be developed.
The molecular pathobiology of ARDS is being extensively defined and the role of several molecules including pattern recognition receptors present on the immune cells (e.g., Toll-like receptors, Nod-like receptors, inflammasomes, and downstream signaling molecules such as NF-κB) and effector molecules (e.g., TNF-α, IL-1β, and IL-18) are being investigated in the pathogenesis of ALI and ARDS. Targeting central molecules such as NF-κB attenuates lung inflammation but has major limitations because inhibition of NF-κB is immunosuppressive and compromises host defense [22]. However, due to the complex nature of the disease, targeting single cytokines or chemokines has also failed to attenuate lung inflammation as these are not sufficient singly to attenuate lung inflammation in ARDS.
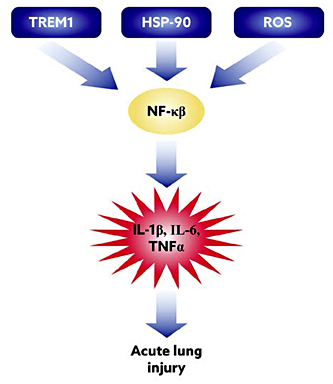
Thus, we have proposed innovative approaches that involve: (1) using GLP-1 peptides as an anti-inflammatory target; (2) targeting upstream molecules such as TREM1 [23,24] and reactive oxygen species and Hsp90 that lead to activation of NF-κB, ultimately leading to ALI and ARDS, with poor outcomes in many cases (Fig. 1); and (3) developing a novel approach to deliver inhibitors of these molecules in vivo. We have shown that the individual nanoformulations are effective at attenuating lung inflammation in a murine model [8,17,18,20].
Potential pathways involved in pathogenesis of lung injury: sequence of events that lead to lipopolysaccharide-induced acute lung injury and key inflammatory pathways we have targeted is depicted.
Potential pathways involved in pathogenesis of lung injury: sequence of events that lead to lipopolysaccharide-induced acute lung injury and key inflammatory pathways we have targeted is depicted.
Sterically Stabilized Phospholipid Nanomicelles (SSM) for Targeted Therapy
Sterically stabilized phospholipid nanomicelles (SSM) are novel, long-acting, biocompatible, and biodegradable phospholipid-based drug delivery vehicles that act as a versatile carrier platform for peptides and water-insoluble drugs. This approach entails self-assembly of distearoylphosphatidylethanolamine covalently linked to polyethylene glycol of molecular weight 2,000 (DSPE-PEG2000) with drugs to form sterically stabilized phospholipid nanomicelles in an aqueous milieu (SSM; size: ∼15 nm in diameter) [13,18]. The nanomicelles are composed of a hydrophilic corona that houses amphipathic peptide drugs, such as TREM1 peptide (Fig. 2, animated) and GLP-1, and a hydrophobic core that accommodates water-insoluble drugs, such as 17-AAG. They are simple to prepare and, unlike liposomes, can be stored in a lyophilized form without lyo- or cryoprotectants for extended periods of time. Nanoparticles can be constructed by various methodologies so that the effect can be targeted at the desired site [6,7,8].
Animated figure showing sterically stabilized phospholipid TREM1 blocking nanomicelles (SSM), composed of a hydrophilic corona that houses amphipathic TREM1 peptide. DSPE, distearoylphosphatidylethanolamine; PEG, polyethylene glycol.
Animated figure showing sterically stabilized phospholipid TREM1 blocking nanomicelles (SSM), composed of a hydrophilic corona that houses amphipathic TREM1 peptide. DSPE, distearoylphosphatidylethanolamine; PEG, polyethylene glycol.
Nanomicelles stabilize peptides in active biological form (α-helix), which is preferred for ligand-receptor interactions, and prevents rapid peptide degradation in vivo, thereby prolonging bioactivity [25,26]. Unlike surfactant micelles, a low critical micellar concentration (∼1 µM) of these nanoparticles prevents their disintegration upon dilution in biological fluids. Importantly, the PEG2000 moiety of SSM confers steric hindrance in the circulation, while their nanosize mitigates renal clearance and extravasation from intact microvessels. This, in turn, prolongs the circulation time of drug-loaded nanomicelles and promotes preferential extravasation from hyperpermeable lung microcirculation, the hallmark of ALI, into the injured lung.
In particular, delivery systems have been shown to increase the stability of a wide variety of therapeutic agents such as hydrophobic molecules, peptides, and oliginucleotides [13,17,18]. These systems are exploited for diagnostic and therapeutic purposes to carry the drug in the body in a controlled manner from the site of administration to the therapeutic target [27]. This implies the passage of the drug molecules and drug delivery system across numerous physiological barriers, which represents the most challenging goal in drug targeting. This innovative, passively targeted therapeutic strategy amplifies drug delivery to the lung, thereby maximizing efficacy and enhancing the resolution of inflammation while reducing collateral damage to innocent bystander organs. These nanomicelles are approved by the FDA for human studies [16,17].
Nanomedicine Therapy for Lung Injury and Acute Respiratory Distress Syndrome
To begin to address the potential of nanotechnology for treatment of ARDS, we developed novel long-acting biocompatible and biodegradable phospholipid micelles (size: ∼15 nm) to modulate key signaling molecules that are critical to the inflammatory response in ALI and ARDS [2,4,21]. We selected molecules that initiate and propagate the inflammatory response by distinct mechanisms so that multiple pathways can be targeted either singly or by a combinatorial approach as in diseases like HIV [28]. Amongst these, TREM1 [23,24,29,30,31,32,33,34], reactive oxygen species, and Hsp90 and GLP-1, a pleiotropic potent anti-inflammatory peptide, were initially selected to modulate the inflammatory response in the lung [4,7,8,17,18].
Realizing the short half-life of peptide drugs (minutes) hampers their clinical use, we invented micellar TREM1 peptide and GLP-1, where each peptide drug is stabilized in its active form (α-helix) and its bioactivity is prolonged for hours in vivo. Likewise, the water insolubility of 17-AAG, a selective Hps90 inhibitor, constrains its use in humans. Accordingly, self-association of 17-AAG with these micelles overcomes this limitation while at the same time increasing its stability and bioavailability. These long-acting micellar drugs provided significant advancements in the treatment of experimental of ALI, which could then be extended to critically ill patients. Nanoparticles can be introduced by systemic administration (oral, dermal, intravenous, etc.) or directly into the lung through inhalation or intranasal or oropharyngeal aspiration. Systemic delivery of nanoparticles is based on the principle of passive targeting. Passive targeting occurs as a result of extravasation of the nanoparticles at the diseased site where the microvasculature is leaky as in ARDS [35].
In vivo Studies to Test Therapeutic Efficacy of SSM
We recently tested the efficacy of GLP-1 nanomicelles in a mouse model of lipopolysaccharide (LPS)-induced lung injury [16,18,36]. Mice (8 weeks old) were treated with LPS nebulization (concentration of 1 mg/mL) administered via a DeVilbiss disposable nebulizer (at a continuous air flow rate of 10 ft3/h) over 1 h to induce ALI. SSM treatment was administered via a subcutaneous route at a dose of 15 nmol of GLP-1/mouse. Control mice were treated with SSM with scrambled peptide or empty SSM. Mice received LPS through nebulization (1 mg/mL). We have previously shown that this model induces neutrophilic influx in the lung. To assess the magnitude of lung inflammation, total and neutrophil cell counts, proinflammatory cytokine levels (TNF-α, IL-6), total and neutrophil counts in bronchoalveolar lavage fluid, and myeloperoxidase activity in lung tissue were measured [16,37]. In vivo administration of GLP-1-SSM to LPS-induced ALI mice resulted in significant downregulation of lung inflammation, with dose-dependent anti-inflammatory activity observed. Similar therapeutic activity was not detected for GLP-1 in saline, indicating that the SSM nanocarriers played a critical role in protecting the enzyme-labile GLP-1 and delivering it to inflamed tissues in vivo. This study demonstrated for the first time that the lipid-based nanoformulation of GLP-1 is effective at attenuating inflammation in ALI/ARDS [16].
We have also tested the efficacy of TREM1 nanomicellar peptides in a model of LPS-induced sepsis and lung injury. TREM proteins are a family of immunoglobulin cell surface receptors expressed on myeloid cells. TREM1 was the first TREM identified, and blockade of TREM1 has been shown to improve survival in animal models of sepsis [33]. We have extensively studied the mechanisms by which TREM1 amplifies inflammation [30,31,32,33,34]. Previous studies where TREM1 blockade was used therapeutically in models of sepsis and infection employed fusion proteins (soluble or extracellular components of TREM1) [23,33]. These peptides are limited by their short half-life and therefore may not be practical for therapeutic purposes.
To effectively block the expression of TREM1, we developed LP17 peptide nanomedicine, LP17-SSM. LP17 is the soluble TREM1 or extracellular component which binds and inhibits signaling through TREM1 [5]. Control SSMs were prepared by using scrambled peptide. For the TREM1 blocking peptide, LP17, circular dichroism confirmed that there was a significant increase in α-helicity in the presence of SSM compared to the free peptides in normal saline. This indicated the presence of peptide-micelle interaction where the peptides changed from a random conformation in saline to a more ordered helical structure when associated with SSM. To determine in vivo anti-inflammatory efficacy and the dose-response effect of LP17-SSM, we established the dose and time-dependent effects as with GLP-1-SSM [16]. Mice were given one dose of LP17 nanomedicine, nanoscrambled peptide, PBS, or LP17 subcutaneously in a single dose prior to LPS challenge. The ALI animal model used was based on previous publications [16,37]. Mice were challenged with aerosolized LPS by nebulization (8 mg LPS dissolved in phosphate-buffered saline at the concentration of 1 mg/mL) administered via DeVilbiss disposable nebulizer (at the continuous air flow rate of 10 ft3/h) over 1 h to induce ALI. The animals were sacrificed 12 h after LPS nebulization.
Firstly we detected the fold change in TREM1 gene induced by LP17 and LP17 nanomedicine in lungs of mice. LP17 inhibited the expression of TREM1; however, there was a significantly lower expression of the TREM1 gene in response to LP17 nanomedicine. Furthermore, the inflammatory parameters including total and neutrophil cell count, cytokines and chemokines, and lung myeloperoxidase were significantly lower in mice that received LP17 nanomedicine compared to other control groups. These data suggest that nanomicellar preparation of TREM1 inhibitory peptide is more efficacious than naked peptide, and can effectively mitigate lung inflammation in LPS-induced lung injury.
Studies with other nanomicellar preparations such as 17-AAG, curcumin, and PPARγ agonists for treatment of ALI/ARDS are currently ongoing in our laboratory [8]. Together, our studies demonstrate the feasibility of translating the use of these nanomicellar preparations for translational human studies to the clinics to treat this devastating disease. We hypothesize that combinatorial administration of nanomicelles or use of more pleiotropic agents that modulate multiple signaling pathways will prove to be more potent; however, further studies are needed to optimize the administration of the nanopreparations.
In vivo Studies Targeting Endothelial Cells to Mitigate Lung Injury
Most of our work has focused on targeting macrophages and immune cells in lung injury. However, the vascular endothelium represents an important therapeutic target for lung injury, especially for systemic conditions such as sepsis. Several studies have focused on targeting the vascular endothelium, especially for molecules that are deficient or overexpressed in lung injury. Studies in animal models have investigated nanocarriers to target endothelial cells [38]. Shuvaev and Muzykantov [39] used vascular immune targeting to modulate reactive oxygen species producing enzymes by encapsulation in protease-resistant carriers. In a mouse model of LPS-induced lung injury, anti-PECAM liposomes loaded with EUK-134, a potent superoxide dismutase/catalase mimetic, enhanced targeting to the endothelium as compared to control IgG-coated liposomes. These liposomes alleviated LPS-induced lung injury, suggesting that anti-PECAM/EUK/liposome may be useful for alleviating acute pulmonary inflammation [40]. Ferrer et al. [41] used dexamethasone encapsulated in ICAM-1-targeted nanogels in a model of pulmonary inflammation. Nanogels are nanosized networks that can absorb large amounts of water while preserving their structure via physical or chemical crosslinks. In contrast to traditional nanoparticles, nanogels can deform to pass physiological filters, resulting in greater delivery efficiency than can be reached using stiffer nanoparticles. By using nanogels, they showed enhanced delivery of DEX to the lungs while reducing the toxicity of free DEX to nontarget organs and showed that the nanogels alleviate pulmonary inflammation. There is scant data that have targeted lung epithelium using a systemic approach. In a mouse model of LPS-induced lung injury, Lin et al. [42 ]tested polyethylenimine and DNA nanoparticles targeted to the β2-adrenergic receptor. Their study showed that treatment with polyethylenimine/ β2-adrenergic receptor improved survival of mice from 28 to 64% in lethal LPS-induced lung injury. Together, these studies show the potential of targeting specific molecules and cell types using a nanomedicine approach to mitigate lung injury and inflammation.
Nanotechnology for Treatment of Infections
Sepsis is the most common cause of ALI and continues to be a major cause of morbidity and mortality. Despite timely and appropriate administration of antibiotics, the host inflammatory response can be overwhelming, leading to multiorgan failure and death [8]. Additionally, there has been an emergence of multidrug-resistant bacteria, which continues to be a pressing threat. Besides antibiotics, alternative approaches to enhance bacterial clearance, such as physical destruction of bacteria or separating bacteria from the blood, are being developed [43]. Microfluidic-micromagnetic separation of bacteria has been studied in vitro in order to develop novel methods to remove bacteria from the bloodstream. It has also been proposed that bacteria can be cleared by physical destruction. In experimental models, such approaches using photodynamic therapy have been shown to destroy bacteria. Lee et al. [44 ]developed an approach for clearing bacteria from the bloodstream by using magnetic nanoparticles modified with a synthetic ligand, zinc-coordinated bis(dipicolylamine). This methodology can be utilized for highly selective and rapid separation of bacteria and their toxins from whole blood using a magnetic microfluidic device.
Nanotechnology for Drug Delivery to the Lung
Microvascular leakiness in ALI and ARDS is the result of increased permeability, and the presence of inflammatory vasoactive factors that enhance permeability [21]. Thus, drugs used for treatment of ALI and ARDS can be administered systemically and will localize to the lungs by passive targeting. This innovative passively targeted therapeutic strategy amplifies drug delivery to the lung, thereby maximizing efficacy and enhancing resolution of inflammation while reducing collateral damage to innocent bystander organs as occurs in patients with ALI and ARDS [8].
Among various drug delivery systems considered for pulmonary application, nanoparticles [45,46,47,48] composed of biodegradable lipid-based nanomicelles and polymers fulfill many requirements placed on these delivery systems, such as the ability to be transferred into an aerosol, stability against forces generated during aerosolization, biocompatibility, targeting of specific sites or cell populations in the lung, release of the drug in a predetermined manner, and degradation within an acceptable period of time [48,49]. Clearly, further studies are warranted to establish the role of aerosolized nanopreparations for inhalational therapy.
Nanoparticle Delivery to the Lung
Most of the currently used delivery systems have low predetermined lung-site deposition efficiencies because some drugs are deposited in the device/spacer and much higher amounts in the oral cavity. Hence, direct drug delivery to the required lung sites has become an active research area. With targeted drug delivery, the respiratory drugs can be efficiently administered to the deeper lung regions of interest without any significant loss in the oral/upper airways. Specifically, such targeted delivery of respiratory drugs using modified inhaler devices can be achieved by controlled release of the drug aerosols from the devices. Hence, there is an urgent need to develop aerosol preparations that can be targeted to the lung. So far we have used nanomicellar preparations for the delivery of targeted drugs to the lungs using a systemic approach. Controlled drug delivery systems have also become increasingly attractive options for inhalation therapies [48,50,51]. The large surface area of the lungs and the minimal barriers impeding access to the lung periphery make this organ a suitable portal for a variety of therapeutic interventions [14,52,53]. The blood barrier between the alveolar space and the pulmonary capillaries is very thin to allow for rapid gas exchange. Alveoli are small and there are approximately 300 million of them in each lung. Although alveoli are tiny structures, they have a very large surface area in total (∼100 m2) for performing efficient gas exchange, making it an attractive organ for direct drug delivery [46,54]. Among the various drug delivery systems considered for pulmonary application, nanoparticles demonstrate several advantages for the treatment of respiratory diseases, such as prolonged drug release, cell-specific targeted drug delivery, or modified biological distribution of drugs, both at the cellular and organ levels [35,39,47,55]. Nanoparticles composed of biodegradable polymers fulfil many requirements placed on these delivery systems, such as ability to be transferred into an aerosol, stability against forces generated during aerosolization, biocompatibility, targeting of specific sites or cell populations in the lung, release of the drug in a predetermined manner, and degradation within an acceptable period of time [49,56,57].
In ALI, intrinsic surfactants are inactivated via albumin leakage and other potential mechanisms. Currently, existing intratracheal surfactants have not proven to be beneficial. By employing novel nanovesicle aerosols of nonlamellar lipids of surfactant, Kaviratna and Banerjee [58] showed that nanopreparation improved the resistance of pulmonary surfactants to inhibition. Further, they showed that nanovesicle aerosols of surfactant were effective in acid-induced lung injury. These represent a novel nanotechnology-based noninvasive therapeutic strategy in ALI and other pulmonary conditions where leakage of inflammatory inhibitor agents forms a major component of the pathophysiology. In a recent study, Zhu et al. [59] developed novel dimethyl silicone dry nanoemulsion inhalations (DSNIs) for pulmonary delivery. The DSNIs showed significantly higher anti-ALI effect on the ALI rat models than the blank DSNIs and the dimethyl silicone aerosols. More recently, Ravikumar et al. [60] developed nanocarrier-based gene therapy to upregulate the expression of pulmonary erythropoietin receptor in an animal model of hyperoxic lung injury. Inhalation delivery resulted in mitigation of acute oxidative lung damage. Together, these studies suggest that aerosolized or inhalational administration of nanocarrier-based therapies may prove to be effective at remedying lung injury.
Optimal delivery of drugs depends on a given patient-specific lung-airway configuration and suitable delivery devices, as well as best aerosol characteristics and inhalation conditions. A suitable delivery device helps in generating drug aerosols of sufficient size and mass to be delivered to the distal lung regions along with the inhaled air/carrier-gas. In recent years, several technical advancements have led to inhalers with efficient drug delivery to localized lung regions. Novel features, such as dose measuring, breath actuation, and predetermined drug release, can significantly improve drug delivery. The development of such drug delivery methodologies, and subsequently device prototyping, require computational fluid-particle dynamic analysis of complex transport phenomena as well as experimental and (ultimately) clinical testing [61,62]. Delivering nanomedicine to predetermined sites of human lung airways is challenging, but offers much promise in combating ALI/ARDS successfully. Observational studies have suggested that the incidence of adverse events is minimized by aerosol delivery; however, there is potential to cause systemic or local toxicity in the form of airway irritation, cough, and often bronchospasms, as well as pulmonary injury when using aerosol therapies [63]. Further research including defining the safety profile of nanoparticles that are delivered via aerosol therapy is necessary. In the future, we anticipate that these nanomicelles will also be applicable to treat other inflammatory lung conditions such as COPD, asthma, bronchiectasis, and pulmonary fibrosis, which are major health concerns in the military and general population. Further studies are needed to establish the role of aerosolized nanopreparations for inhalational therapy.
Conclusion
Despite advances in understanding the pathobiology of ARDS, treatment options for this devastating condition are limited. Nanomedicine, the medical application of nanotechnology, promises an endless range of applications from biomedical imaging to drug delivery and therapeutics. These novel approaches using nanotechnology are revolutionizing the future of medicine. Nanoparticulate drug delivery systems, designed as multifunctional engineered nanoparticles, appear to be particularly attractive and promising for drug delivery to organs such as the lung since they combine several opportunities like uniform distribution of drug dose among all ventilated alveoli, allowing for uniform cellular drug internalization. Besides sustained release of drugs in plasma and organs, other potential advantages of the system include the possibility of reduction in drug dosage, adverse effects, and drug interactions. Inhalational delivery of nanoparticles would also allow for targeted delivery of the drug while minimizing systemic effects. Although the field of nanomedicine offers multiple opportunities, it still is in its infancy and the research has to proceed in order to obtain specific targeting of the drug combined with modes of delivery. Ongoing studies offer several exciting prospects for the application of engineered nanomedicine preparations and aerosol preparation for drug delivery in the years to come.
Acknowledgements
R.T.S. received funding from the US Department of Veterans Affairs and a VA Merit Grant. We thank Dr. Hayat Onyuksel for her invaluable scientific contribution.
Disclosure Statement
The authors declare no conflicts of interest.