Abstract
Patients with severe aortic stenosis (AS) show progressive fibrotic changes in the myocardium, which may impair cardiac function and patient outcomes even after successful aortic valve replacement. Detection of patients who need an early operation remains a diagnostic challenge as myocardial functional changes may be subtle. In recent years, speckle tracking echocardiography (STE) and cardiac magnetic resonance mapping have been shown to provide complementary information for the assessment of left ventricular mechanics and identification of subtle damage by focal or diffuse myocardial fibrosis, respectively. Little is known, however, about how focal and diffuse myocardial fibrosis occurring in severe AS are related to measurable functional changes by echocardiography and to which extent both parameters have prognostic and diagnostic value. The aims of this review are to discuss the occurrence of focal and diffuse myocardial fibrosis in patients with severe AS and to explore their relation with myocardial function, determined by STE, as well as the prognostic and diagnostic potential of both parameters.
Introduction
The appropriate timing of aortic valve replacement (AVR) in asymptomatic patients with severe aortic stenosis (AS) remains challenging [1, 2]. Several of these patients show progressive fibrosis of the left ventricular (LV) myocardium, which may impair cardiac function and clinical outcomes even after successful AVR [3-5]. These individuals may benefit from early AVR before the development of irreversible myocardial fibrosis. The identification of myocardial damage at an early stage remains challenging. Indices provided by standard echocardiography show a low sensitivity as myocardial structural and functional changes may be subtle. Cardiac magnetic resonance (CMR) and speckle tracking echocardiography (STE) have been recently shown to provide complementary information in the assessment of myocardial fibrosis and its functional consequences, respectively [6-9]. However, information on the clinical value of the use of these cardiac imaging techniques in valvular heart disease is scant. Moreover, little is known about the relationship between myocardial fibrosis and measurable LV systolic function by STE. Accordingly, the aim of the present paper is to review the existing scientific literature on the relation between myocardial fibrosis and LV dysfunction and its possible impact on clinical outcomes in patients with AS.
Pathophysiology of LV Dysfunction in AS
Obstruction of the LV outflow tract due to AS is associated with a gradual increase in the LV afterload, which ultimately leads to the development of LV hypertrophy. Until recently, LV hypertrophy in AS had been considered a compensatory mechanism of the left ventricle muscle to face the high-pressure overload. Hypertrophied LV is capable of generating greater forces and higher pressures, while the increased wall thickness maintains a normal wall stress and sustains LV contractions. However, this original view of LV hypertrophy as a solely compensatory process has changed in the last decades. Focused papers have in fact demonstrated a significant relationship between LV hypertrophy and increased LV stiffness, diastolic dysfunction, and increased LV filling pressure [10-12]. Thanks to recent advances in cardiac imaging, a close association has been observed between the development of LV hypertrophy and myocardial fibrosis [13]. It has been postulated that, while originally being a compensatory process, LV hypertrophy ultimately becomes maladaptive and leads to myocyte apoptosis and diffuse interstitial myocardial fibrosis. These changes make the cardiac muscle less compliant and are responsible for the progression of LV hypertrophy towards overt heart failure [14-16]. Cardiac fibrocyte cells normally produce collagen to provide structural support for the heart. When overactivated in response to pressure overload, this process causes excessive accumulation of fibrosis and damages myocardial muscles. In histology, 2 types of myocardial fibrosis have been described: diffuse myocardial fibrosis (DMF), an early form of fibrosis believed to be reversible, and focal myocardial fibrosis (FMF), a later form that is irreversible [17]. AS is characterized by a significant increase in DMF, with a large variation in interindividual values [6, 17]. The extent of DMF has been shown to be an independent predictor of adverse clinical outcomes both before and after AVR as well [15, 18, 19]. Notably, patients with paradoxical low-flow low-gradient AS have a higher degree of myocardial fibrosis and LV longitudinal dysfunction than patients with normal-flow high-gradient AS [16, 20]. It has been hypothesized that not only a reduced LV cavity but also LV functional changes as a consequence of myocardial fibrosis contribute to a reduction in the LV stroke volume and production of a low transvalvular gradient, thus leading to a poor outcome [20, 21]. This suggests that DMF may be one of the critical mechanisms underlying the transition of LV hypertrophy to heart failure with an unfavorable clinical course. Accordingly, an accurate diagnostic technique, able to assess DMF or its functional correlates, may be crucial in patients experiencing AS.
Imaging of Diffuse Myocardial Fibrosis in AS
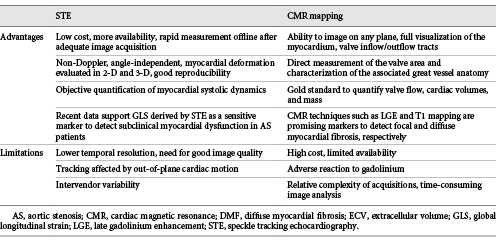
LV myocardial biopsy has been the gold standard for evaluation of DMF for a long time. However, the invasiveness, susceptibility to sampling errors, and inability to assess the fibrotic burden of the whole LV myocardium hamper its clinical utility in daily practice. CMR has emerged as a reference noninvasive method to assess both FMF and DMF [6, 15, 26]. Late gadolinium enhancement (LGE) at CMR is an established technique for assessing FMR (replacement fibrosis, scar). In symptomatic patients with severe AS, FMF occurs mainly in the subendocardial layer of the LV and its degree decreases from the base to the apex [15, 16]. Patients with a larger extent of FMF had a significantly lower freedom from cardiac death at 10 years (42 ± 19% vs. 89 ± 6%, p = 0.002), with congestive heart failure being the most common cause of death [3]. In another study, the presence of FMF was significantly associated with poor postoperative outcomes [17]. However, FMF develops later in the disease course and, therefore, CMR-derived LGE is not sensitive enough to detect the early stage of myocardial damage. Accordingly, in our previous studies which used CMR-derived T1 mapping (CMR-T1), a total of 25% of patients had extensive (> 30%) DMF and a focal scar was not observed in any of them [23, 24]. Using the MOLLI sequence, CMR-T1 was in fact recently shown to allow accurate detection and quantification of DMF with excellent precision, reproducibility, and scan-rescan stability [22]. The T1 mapping technique measures the myocardial T1 relaxation time before or after contrast administration. An increased collagen content with expansion of the extracellular space causes prolongation of the native T1 relaxation time and an extracellular volume (ECV) fraction increase in comparison with normal myocardium. Both native T1 relaxation time and ECV have been significantly associated with DMF at myocardial histology [25-27]. We recently reported the high accuracy of both native T1 relaxation time with a cut-off value ≥1,010 ms (Ss = 90%, Sp = 73%, AUC = 0.82) and ECV with a cut-off value ≥0.315 (Ss = 80%, Sp = 90%, AUC = 0.85) to identify extensive (> 30%) DMF at histology [24]. Moreover, correlations between both native T1 and ECV with prognostic markers such as NT-pro-BNP or troponin have been reported [28, 29]. CMR-T1 has therefore been proposed as a promising technique to identify early structural changes in patients with AS. The advantages and limitations of CMR in AS assessment are shown in Table 1.
Imaging of Early LV Dysfunction in AS
LV ejection fraction by echocardiography is routinely used to assess LV systolic chamber function in patients with AS. However, increasing evidence demonstrates that irreversible myocardial damage might occur before changes in the ejection fraction become apparent [8]. It is noteworthy that AS-induced DMF starts at the subendocardial level, affecting mainly longitudinal LV function. Since it is predominantly determined by radial function, the LV ejection fraction can be normal for a long time even in the presence of extensive subendocardial fibrosis [6, 15, 19]. Accordingly, the LV ejection fraction, i.e., the class I guideline recommendation for AVR, cannot be used for early risk stratification in asymptomatic AS patients. In contrast, STE-derived 2-D global longitudinal strain (GLS) is a validated and sensitive parameter to quantify LV longitudinal systolic function [8, 9]. Several studies have demonstrated a reduced magnitude of GLS in AS patients compared to controls despite a preserved LV ejection fraction [16-18, 31-33]. In asymptomatic AS, GLS at rest has been shown to be independently associated with development of symptoms, an abnormal exercise tolerance, a need for AVR, and mortality [34-37]. Furthermore, a magnitude of the longitudinal strain of LV basal segments below −13% has been found to be associated with a higher rate of cardiac events at follow-up [32]. It has also been shown that a GLS below −18% predicts an abnormal exercise response with a sensitivity of 68% and a specificity of 77% [38]. In another study, the assessment of GLS during exercise had a higher accuracy than the LV ejection fraction to detect latent LV systolic dysfunction [39]. Finally, even the decrease in circumferential strain may be a marker of advanced disease with unfavorable course, particularly when it is associated with a low-flow state in AS patients [40]. These findings suggest that both regional and GLS have a greater and earlier diagnostic power than the LV ejection fraction in this clinical setting [41]. The advantages and limitations of the STE-derived GLS assessment are summarized in Table 1.
Relationship between Myocardial Fibrosis and LV Systolic Function
Different kinds of observations have shown that GLS is a functional marker of myocardial fibrosis. First of all, GLS was found to be related to biomarkers of myocardial fibrosis such as those expressing calcification, collagen formation, or breakdown and inflammation [42, 43]. Several studies have also reported significant associations between LV systolic function and both FMF and DMF at CMR or myocardial histology [3, 14, 15, 18, 19, 24, 29, 44-46] (Table 2). Former studies have investigated the relationship between FMF and LV contractile function [14, 44, 45]. It has been shown that both the presence and the extent of FMF are inversely related to echocardiographic parameters such as relative wall thickness, LV fractional shortening, and ejection fraction and to STE-derived indices of LV myocardial function [18, 28, 44]. A GLS ≤−11.6% showed a sensitivity of 65% and a specificity of 75% to predict significant FMF (LGE > 10%) [43]. The majority of studies dealing with this issue have focused on DMF [15, 19, 24, 46, 47]. Of the conventional echocardiography-derived parameters, DMF seems to show a significant, though weak, correlation only with LV mass and the LV mass index [23, 24]. In contrast to FMF, none of the other conventional parameters including LV ejection fraction or aortic valve area had a significant association with the degree of DMF [39]. This emphasizes the need to use a highly sensitive technique to assess DMF. Recent investigations have reported a significant relationship among DMF at histology, the CMR-T1-derived native T1 relaxation time or ECV, and STE-derived deformation indices [15, 18, 45]. In our study, a GLS <–15% showed excellent accuracy to predict extensive (> 30%) DMF (Fig. 1, 2) [23, 24]. Moreover, we observed a significant correlation between GLS during exercise and native T1 relaxation time (Fig. 3) [23, 24]. Finally, the native T1 relaxation time showed a high accuracy in predicting the limited LV contractile reserve [23, 24]. All together these results strongly support the concept that GLS could be considered as an accurate functional marker of DMF in AS.
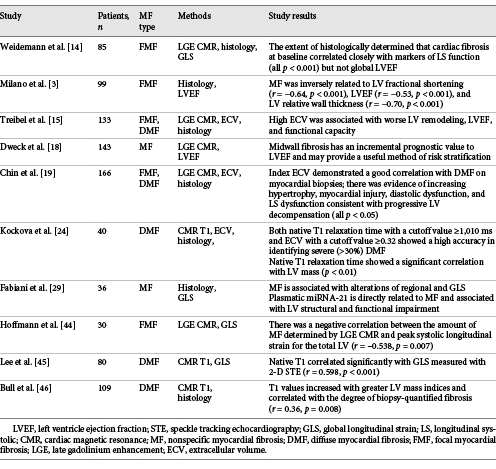
Studies showing relationships between myocardial fibrosis and LV systolic function assessed by different methods
Examples of resting 2-D GLS compared with the extent of DMF on myocardial histology. a Patient with a preserved magnitude of 2-D GLS (–21.1%) and a negligible extent of DMF (7.4%). b Patient with a reduced magnitude of 2-D GLS (–14.9%) and extensive DMF (31.2%). DMF, diffuse myocardial fibrosis; GLS, global longitudinal strain. The images are shown with permission from the research work group of the Cardiovascular Center Aalst (Belgium) [23, 24].
Examples of resting 2-D GLS compared with the extent of DMF on myocardial histology. a Patient with a preserved magnitude of 2-D GLS (–21.1%) and a negligible extent of DMF (7.4%). b Patient with a reduced magnitude of 2-D GLS (–14.9%) and extensive DMF (31.2%). DMF, diffuse myocardial fibrosis; GLS, global longitudinal strain. The images are shown with permission from the research work group of the Cardiovascular Center Aalst (Belgium) [23, 24].
a Correlation between 2-D GLS and the percentage of myocardial collagen on myocardial histology. b Accuracy of resting 2-D GLS to identify extensive (> 30%) DMF on myocardial histology. DMF, diffuse myocardial fibrosis; GLS, global longitudinal strain. The images are shown w permission from the research work group of the Cardiovascular Center Aalst [23, 24].
a Correlation between 2-D GLS and the percentage of myocardial collagen on myocardial histology. b Accuracy of resting 2-D GLS to identify extensive (> 30%) DMF on myocardial histology. DMF, diffuse myocardial fibrosis; GLS, global longitudinal strain. The images are shown w permission from the research work group of the Cardiovascular Center Aalst [23, 24].
a Correlation between exercise-induced Δ 2-D GLS and native T1 relaxation time on a 3-T scan. b Accuracy of native T1 relaxation time on a 3-T scan to predict a reduced LV contractile reserve. DMF, diffuse myocardial fibrosis; GLS, global longitudinal strain. The images are shown with permission from research work group of the Cardiovascular Center Aalst [23, 24].
a Correlation between exercise-induced Δ 2-D GLS and native T1 relaxation time on a 3-T scan. b Accuracy of native T1 relaxation time on a 3-T scan to predict a reduced LV contractile reserve. DMF, diffuse myocardial fibrosis; GLS, global longitudinal strain. The images are shown with permission from research work group of the Cardiovascular Center Aalst [23, 24].
Limitations
Although both CMR-T1 and STE seem to have great clinical potential in various cardiovascular diseases, these techniques also have several limitations (Table 1). One of the major shortcomings of both methods is the great interscanner or intervendor variability of normal values. This disadvantage requires definition of normal values for each individual scanner or echo device when assessing healthy subjects. This procedure should be repeated after each major update of equipment or hardware. Other limitations need also mentioned. First of all, CMR-derived assessment of FMF using LGE has a wide interobserver variability, depends on the technical setting of the scanner, and does not allow detection of DMF [47]. The CMR-T1-derived T1 relaxation time and ECV are dependent on a specific CMR-T1 sequence, magnetic field strength, and homogeneity. In addition, there is a significant overlap between T1 mapping values in healthy and diseased myocardia, making the interpretation challenging [15, 30, 39, 40]. Other limitations of CMR include the limited availability of equipment and expertise, the associated high costs, and the need to administer a contrast agent. In contrast, echocardiography is more widely available, faster, and cheaper than CMR. GLS, a relatively operator-independent parameter, has a higher reproducibility compared to LV ejection fraction and other echocardiographic parameters of LV systolic function [6]. However, due to the difference among different vendors, the same software should be used in individual patients over time [48-50]. The load dependency of the STE-derived indices may represent another challenge for routine clinical use in AS, as they are largely influenced by both preload and afterload changes [27, 38, 39, 51]. According to recent published studies in animal models, STE-derived indices correlate strongly with pressure-volume loop-derived contractility indices and the STE-derived strain cannot predict load-independent contractility [51, 52]. Accordingly, to bypass this limitation in the chronic overloaded LV, the pressure-strain loop-based method is a promising tool for assessment and monitoring of myocardial function in patients with AS, but this method is still under investigation. Recently, novel techniques of derived tissue tracking by CMR cine acquisitions, such as CMR tagging and feature tracking, have provided a detailed characterization of LV global and regional contractility and reasonable agreement in the assessment of myocardial deformation in patients with AS [53-55]. However, several technical limitations may affect quantitative results and lead to variability among different readers [56-58]. Finally, the role of tissue tracking by CMR in detection of the extent and types of myocardial fibrosis could be compromised by the coexistence of other comorbidities, such as hypertension, amyloidosis, or ischemic heart disease, which may play a role in disease phenotyping [59, 60]. Thus, the accuracy of these emerging methods for characterization of LV performance and quantification of myocardial fibrosis in patients with isolated AS or a concomitant comorbidity is still not adequately identified [61-64].
Conclusions
There is growing evidence that myocardial fibrosis plays an important role in the pathophysiology of AS and its complications. Recent advances in cardiac imaging technology allow noninvasive detection of myocardial fibrosis and the associated impairment of LV systolic function. It has been demonstrated that evaluation of myocardial fibrosis by CMR and of its functional consequences highlighted by GLS provides a more accurate assessment of early myocardial damage than LV ejection fraction. Despite its great diagnostic potential, further improvement of the current technology is needed to homogenize CMR-T1- and STE-derived indices across different vendors and scanners. Future advances in noninvasive cardiac imaging might improve our understating of the interplay between myocardial fibrosis and LV function. The real clinical value of these parameters reflecting early myocardial injury needs to be validated in multicenter prospective studies.
However, the encouraging results derived from different studies provide clinical perspectives on the use of these techniques for guidance in clinical decision making and improvement of the management of patients with AS.
Acknowledgement
Dr. Katbeh was supported by a research grant from the International PhD programme in Cardiovascular Pathophysiology and Therapeutics (CardioPaTh).
Disclosure Statement
The authors have no conflicts of interest to declare.



![Fig. 1. Examples of resting 2-D GLS compared with the extent of DMF on myocardial histology. a Patient with a preserved magnitude of 2-D GLS (–21.1%) and a negligible extent of DMF (7.4%). b Patient with a reduced magnitude of 2-D GLS (–14.9%) and extensive DMF (31.2%). DMF, diffuse myocardial fibrosis; GLS, global longitudinal strain. The images are shown with permission from the research work group of the Cardiovascular Center Aalst (Belgium) [23, 24].](https://karger.silverchair-cdn.com/karger/content_public/journal/crd/141/3/10.1159_000493164/2/m_000493164_f01.jpeg?Expires=1714527186&Signature=090onCH5WRt89wy5mG~9nNkpHh0XW0o8UR23BFuxOlzANW~Lf5CxKmdVeJAUNUBvSSREgeM-ScU4W93X2lbRY9awBPWSdDcmUwVBKUAiCot-pd7BWiXeD4WpzIEUFVVn9Kt9BuN68LOhJDJjIrRauPG9QWF4Cjb9CkBvjB5fmTMRo07QIyZSyrrZyvVLENmx~t74wg~655Q-awajaJs7n2skuBUJDGGqswAxXWXU1gkk7VEgFEOKVxgH08S9Ffn3jvFedgfXyjGoitBOM-YEucX9FDZUyLfEwoPm0CpweRj2NQ7mnK-ZN1pCqjH67LVoBTxMp41QqCgXp~U5wg0ijA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. a Correlation between 2-D GLS and the percentage of myocardial collagen on myocardial histology. b Accuracy of resting 2-D GLS to identify extensive (> 30%) DMF on myocardial histology. DMF, diffuse myocardial fibrosis; GLS, global longitudinal strain. The images are shown w permission from the research work group of the Cardiovascular Center Aalst [23, 24].](https://karger.silverchair-cdn.com/karger/content_public/journal/crd/141/3/10.1159_000493164/2/m_000493164_f02.jpeg?Expires=1714527186&Signature=ezGdWsdySCFtVgPgCVSjeLziiX~lJ5iQK0h4kGifqwMJG40O3yFPUT9pCFfYjbawG8JUDNHAnKgw2gXOnhs7ffzd3csfKtLPxxzFbXsddF7i6FT8ElhNsBK6qkiKqXdNmnN2vV8sNz7s~CfXhBSN9Z6DOxfqDEkmUYnAiktsVvIty8Ig-QZx6XN~KtPDB6rDURy2oFFVAAfuALfoEtgxGSADST8dKpXSdR5x4X8fXinEJiNA6dcNLtQh3v6cm~fGq-bPZ0~oJGjfjk2SkVwPAeQmo9BbQAeEBDnhjxu~Mkqhq1YgHqa3-rwq7kph8dOlXK6VogyAPKUOtR9cFM-koA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. a Correlation between exercise-induced Δ 2-D GLS and native T1 relaxation time on a 3-T scan. b Accuracy of native T1 relaxation time on a 3-T scan to predict a reduced LV contractile reserve. DMF, diffuse myocardial fibrosis; GLS, global longitudinal strain. The images are shown with permission from research work group of the Cardiovascular Center Aalst [23, 24].](https://karger.silverchair-cdn.com/karger/content_public/journal/crd/141/3/10.1159_000493164/2/m_000493164_f03.jpeg?Expires=1714527186&Signature=u4mMSB0EPLSWiAdifQ~NLK954jO7~~9njySAMbBAE5quYwtpRESY6jtwhd5IKE-83P7g5DhXhN4Axg72Qee3tipsHURxN414gxjZRcRNalgWY06C9vdoXyUMJDX4omE9OPF2QuKc7Coinu7-uhmWYfsz8KlLC5xe52TfsW~q9547jsByjVLH3zLDzY2wvyuVw-VPyNJOLBjpG~ajyrV1tBMYTzdihlqyPGbDAXthzyqEnqY2hloOQKyeezadS9Rz-B1IkyRK0SdiJOOIfCe-vd4PpV-BOwNwiFMkX7ni0r~717OX5qybSAIiveI4kwF6xM3~rh5lyLsp30NNxKK-tw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)