Abstract
Cancers of the gastrointestinal tract, including the liver, bile ducts, and pancreas, constitute the largest group of malignant tumors. Colorectal cancer is one of the most common neoplastic diseases in Western countries and one of the leading causes of cancer-related deaths. Inactivation of the adenomatous polyposis coli (APC) tumor-suppressor gene during early adenoma formation is thought to be the first genetic event in the process of colorectal carcinogenesis followed by mutations in oncogenes like K-Ras and tumor-suppressor genes like p53. Identification of the interaction of APC with the proto-oncogene β-catenin has linked colorectal carcinogenesis to the Wnt-signal transduction pathway. The main function of APC is thought to be the regulation of free β-catenin in concert with the glycogen synthase kinase 3β (GSK-3β) and Axin proteins. Loss of APC function, inactivation of Axin or activating β-catenin mutations result in the cellular accumulation of β-catenin. Upon translocation to the nucleus β-catenin serves as an activator of T-cell factor (Tcf)-dependent transcription leading to an increased expression of several specific target genes including c-Myc, cyclin D1, MMP-7, and ITF-2. While APC mutations are almost exclusively found in colorectal cancers, deregulation of Wnt/β-catenin/Tcf signaling is also common in other gastrointestinal and extra-gastrointestinal human cancers. In a fraction of hepatocellular carcinomas the Wnt pathway is deregulated by inactivation of Axin or stabilizing mutations of β-catenin. The majority of hepatoblastomas and a group of gastric cancers also carry β-catenin mutations. Clearly, this pathway harbors great potential for future applications in cancer diagnostics, staging, and therapy.
Introduction
The Wnt pathway plays key roles in development, tissue homeostasis, and cancer [1, 2, 3, 4, 5]. It was originally described in Drosophila as Wingless pathway and is highly conserved among flies, frogs, and mammals. The combined effort of genetic, biochemical and developmental research has led to the comprehensive understanding of the Wnt pathway as it is known today. The most extensively studied part of this pathway leads to transcriptional activation of specific genes and is referred to as the canonical Wnt pathway (fig. 1): Extracellular Wnt proteins bind to and activate membrane-bound Frizzled receptors which in turn mediate phosphorylation of Dishevelled. Through binding to Axin Dishevelled inhibits phosphorylation of β-catenin by disrupting a complex consisting of the adenomatous polyposis coli (APC), Axin, and glycogen synthase kinase 3β (GSK-3β) proteins. Unphosphorylated β-catenin then binds to transcription factors of the T-cell factor/lymphoid-enhancer factor (Tcf/Lef) family and activates the transcription of specific target genes including c-Myc, cyclin D1, MMP7, gastrin and ITF-2 [6, 7, 8, 9, 10, 11]. However, binding of some Wnt factors to certain Frizzled receptors can also result in Ca2+ release and protein kinase C activation, which does not lead to activation of β-catenin/Tcf signaling. In contrast to the canonical Wnt pathway this signal transduction cascade has been named the Wnt/Ca2+ pathway [12].
Wnt-signaling and regulation of free β-catenin. a Under physiological conditions the largest fraction of β-catenin (β) is bound to the cell–cell adhesion molecule E-cadherin (E-cad). Thereby it links the cell membrane via α-catenin (α) to the actin cytoskeleton. After priming phosphorylation by CKI, free β-catenin is bound by the APC/Axin/GSK complex and phosphorylated amino terminally. Phosphorylated β-catenin binds to the F-box protein β-TrCP and, after ubiquitination (Ub), is degraded by the proteasome. b Binding of Wnt ligands to a serpentine Frizzled receptor (Frz) results in phosphorylation of Dishevelled (Dsh). Upon binding to Axin the APC/Axin/GSK-complex dissociates and β-catenin bypasses the destruction machinery. After translocation to the nucleus β-catenin binds to T-cell factors (Tcf) and recruits the chromatin-remodeling proteins p300 and Brg-1 to responsive promoters, thereby activating the transcription of specific target genes, including c-Myc, cyclin D1, matrilysin, gastrin, and ITF-2. Soluble Frizzled-related proteins (sFRP) bind to Wnt factors and exert a Wnt-antagonistic effect.
Wnt-signaling and regulation of free β-catenin. a Under physiological conditions the largest fraction of β-catenin (β) is bound to the cell–cell adhesion molecule E-cadherin (E-cad). Thereby it links the cell membrane via α-catenin (α) to the actin cytoskeleton. After priming phosphorylation by CKI, free β-catenin is bound by the APC/Axin/GSK complex and phosphorylated amino terminally. Phosphorylated β-catenin binds to the F-box protein β-TrCP and, after ubiquitination (Ub), is degraded by the proteasome. b Binding of Wnt ligands to a serpentine Frizzled receptor (Frz) results in phosphorylation of Dishevelled (Dsh). Upon binding to Axin the APC/Axin/GSK-complex dissociates and β-catenin bypasses the destruction machinery. After translocation to the nucleus β-catenin binds to T-cell factors (Tcf) and recruits the chromatin-remodeling proteins p300 and Brg-1 to responsive promoters, thereby activating the transcription of specific target genes, including c-Myc, cyclin D1, matrilysin, gastrin, and ITF-2. Soluble Frizzled-related proteins (sFRP) bind to Wnt factors and exert a Wnt-antagonistic effect.
70–80% of colorectal cancers have defects in the Wnt pathway [13, 14]. Most frequently APC mutations are found, but a subset of tumors wild-type for APC carry β-catenin mutations. The current model of colorectal carcinogenesis predicts that at least four mutations in critical genes are required for the evolution of colorectal cancer [15, 16]. The earliest adenomatous stages are associated with inactivating APC mutations, followed by mutations of the oncogene K-Ras and inactivation of tumor-suppressor genes including p53. Roughly 20–30% of hepatocellular carcinomas (HCCs) carry mutations in the Axin or the β-catenin gene [5]also resulting in deregulation of Wnt signaling. The epidemiological importance of cancers associated with defects in the Wnt pathway is evident. Colorectal cancer is one of the leading causes of cancer-related morbidity and mortality in Western countries. In Europe alone more than 210,000 new cases and 110,000 deaths are reported each year [17]and the risk of developing colorectal cancer during a lifetime is about 5–6%. While colorectal cancer is much more common in developed than in developing countries, HCC is the most frequent cancer in some regions of the world [17].
Components of the Wnt Pathway and Their Contribution to Gastrointestinal Tumors
The First Steps in the Wnt-Signaling Cascade:Wnt Factors, Frizzled Receptors and Dishevelled
Wnt proteins constitute a large family of at least 16 secreted cysteine-rich glycoproteins, some of which have been shown to promote neoplastic transformation in animal models and tissue culture systems [18]. However, in contrast to down-stream components of the pathway, their direct involvement in human carcinogenesis has never been demonstrated. Wnt proteins bind to the extracellular domain of the Frizzled family of seven transmembrane receptors. So far more than 11 different Frizzled genes have been identified in vertebrates, but little is known about their specific functions and ligand specificities. Recently, the low-density lipoprotein receptor-related proteins, LRP5 and LRP6, have been found to act as co-receptors for Wnt signal transduction [19, 20, 21]. In addition to membrane-bound Frizzled receptors, a group of secreted Frizzled-related proteins has been described. Through binding of Wnt proteins they seem to exert an antagonistic effect on Wnt signaling [22, 23, 24]. However, some of the Frizzled-related proteins also seem to bind to classical membrane-bound Frizzled receptors [25]and some even seem to activate Wnt signaling [26]. Whether Frizzled receptors or Frizzled-related proteins directly contribute to carcinogenesis is not clear yet, but it has been reported that the Frizzled receptor E3 (FzE3) is expressed in many esophageal cancers but not in matched normal tissues [27]. Interestingly, this expression of FzE3 correlates with nuclear translocation of β-catenin.
Binding of a Wnt ligand to a member of the Frizzled receptor family results in its activation. Activated Frizzled receptors recruit the cytoplasmic protein Dishevelled to the inner cell membrane and mediate its phosphorylation [28, 29, 30, 31]. It is unknown, whether Dishevelled directly binds to Frizzled receptors or whether its binding is mediated through other so far unknown proteins. To date, two main functions of Dishevelled have been identified. Through distinct domains Dishevelled transduces Wnt signals [32]and activates the jun-N terminal kinase (JNK) pathway [33, 34, 35]. Wnt signals are transduced by direct binding of Dishevelled to Axin [30, 36]. This results in the inhibition of GSK-3β-dependent phosphorylation of β-catenin. Most likely this occurs through disintegration of the APC/Axin/GSK-3β complex [36]. In addition, protein kinase CK2 (casein kinase II), a protein serine/threonine kinase, is also able to phosphorylate Dishevelled independent of Frizzled [37, 38]. How far Dishevelled and CK2 directly promote neoplastic transformation via β-catenin/Tcf is unknown. So far no mutations of these proteins have been reported in human cancers.
GSK-3β, Axin, Casein Kinase I, and β-TrCP: Regulators of β-Catenin
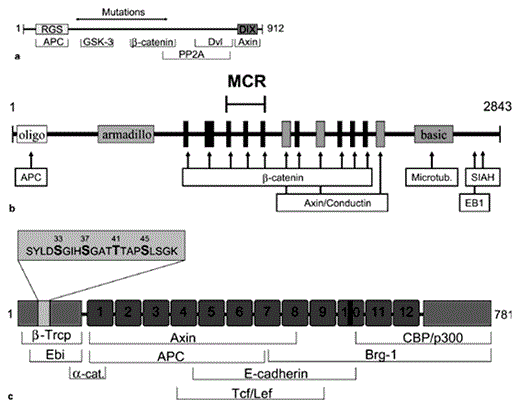
Tight regulation of the free cytoplasmic pool of β-catenin is the central switch of the Wnt pathway. The current view predicts that in the absence of a Wnt-signal degradation of β-catenin is initiated by priming phosphorylation of serine residue 45 (S45) by casein kinase I (CKI) [39, 40]. Phosphorylation of S45 is dependent on binding of CKI to axin [40]. The next step involves a multi-protein complex consisting of β-catenin, APC, Axin, and the serine/threonine kinase GSK-3β [13]. In this complex GSK-3 facilitates further phosphorylation of β-catenin’s amino terminus: starting at threonine 41, and walking downstream to S37 and S33. The aim of this cascade is the generation of the canonical β-TrCP recognition site around S33/S37 (DS*GXXS*; S* = phosphoserine; fig. 2c) [41]. Then β-catenin isoforms phosphorylated at all four critical serine/threonine residues are bound by the F-box protein β-TrCP [42, 43, 44], a subunit of the SCF-type E3 ubiquitin ligase complex [45]. This complex facilitates ubiquitination and subsequent proteasome degradation of phosphorylated β-catenin [46]. The finding that priming phosphorylation of S45 is required for further phosphorylation by GSK-3β solves the longstanding mystery why mutation of one of the four serine and threonine residues is sufficient for inhibition of β-catenin degradation: mutation of one single of the four phosphorylation targets inhibits further downstream phosphorylation. And phosphorylation of serines 33 and 37 is required for binding of β-catenin to β-TrCP. In addition to mutations of the aforementioned serine and threonine residues, mutations of asparagin 32 or glycin 34, which are also common in human cancers [5], likewise destroy the β-TrCP recognition motif site, resulting in stabilization of β-catenin. In contrast, mutation of serines 25 and 29 does not affect the stability of β-catenin since both residues are located amino terminally to the β-TrCP-binding region. The significance of these mutations for carcinogenesis remains to be determined [47].
Components of the Wnt pathway found to be mutated in gastrointestinal cancers. a Mutation of Axin-1 in hepatocellular carcinoma results in loss of regions responsible for binding to GSK-3 and β-catenin. b APC forms homodimers with itself by the oligomerization domain (oligo). The armadillo repeat region is made up of a 42-amino acid motif that is repeated 13 times. The regions for binding and downregulation of β-catenin and the binding region of Axin/conductin partly overlap. Microtubules bind to the basic region (basic) and the binding regions of Siah-1 and EB1 are confined to the carboxy terminus. The mutation cluster region (MCR) is located amino terminal to the Axin/conductin-binding sites. Most mutant APC proteins can no longer bind to Axin and are therefore incapable of downregulating β-catenin. c The amino and carboxy termini of β-catenin serve as transcriptional activators. The central part is made up of 12 highly homologous armadillo repeats (boxes 1–12) which mediate most interactions with other proteins. Serine and threonine residues 33, 37 and 41 (insert) are the GSK-3 phosphorylation sites. Serine 45 is the target of priming phosphorylation by CKI. Mutation of one of these residues prevents degradation of β-catenin.
Components of the Wnt pathway found to be mutated in gastrointestinal cancers. a Mutation of Axin-1 in hepatocellular carcinoma results in loss of regions responsible for binding to GSK-3 and β-catenin. b APC forms homodimers with itself by the oligomerization domain (oligo). The armadillo repeat region is made up of a 42-amino acid motif that is repeated 13 times. The regions for binding and downregulation of β-catenin and the binding region of Axin/conductin partly overlap. Microtubules bind to the basic region (basic) and the binding regions of Siah-1 and EB1 are confined to the carboxy terminus. The mutation cluster region (MCR) is located amino terminal to the Axin/conductin-binding sites. Most mutant APC proteins can no longer bind to Axin and are therefore incapable of downregulating β-catenin. c The amino and carboxy termini of β-catenin serve as transcriptional activators. The central part is made up of 12 highly homologous armadillo repeats (boxes 1–12) which mediate most interactions with other proteins. Serine and threonine residues 33, 37 and 41 (insert) are the GSK-3 phosphorylation sites. Serine 45 is the target of priming phosphorylation by CKI. Mutation of one of these residues prevents degradation of β-catenin.
Besides β-catenin, GSK-3β also phosphorylates other members of the Wnt pathway, including Axin [48]and APC [49], thereby regulating the stability of Axin and the binding efficiency of APC to β-catenin, respectively. For phosphorylation of β-catenin by GSK-3β the presence of Axin is required [50, 51]. Axin, or its homolog conductin (also called Axil or Axin-2), serves as a scaffold protein allowing assembly of the APC/Axin/GSK-3β/β-catenin complex [52]. Interaction of Axin proteins with APC, GSK-3β, and Axin as well as with Dishevelled occurs by non-overlapping regions. Binding to APC is facilitated through the RGS domain [53, 54]and binding to Dishevelled occurs through a domain called DIX which is similar to a region also found in Dishevelled [30, 55](fig. 2a). Based on its function to downregulate oncogenic Wnt signaling Axin could be viewed as a tumor-suppressor gene. In fact, this view is supported by several findings. In a subset of HCCs Axin is biallelically mutated leading to Axin proteins lacking the β-catenin-binding site [56]. And in 11 of 45 colorectal cancers with defective DNA mismatch repair, lacking mutations in both β-catenin and APC, the Axin-2 gene was found to be mutated [57]. Interestingly, Axin-2 was just recently identified to be a target gene of β-catenin/Tcf signaling (see below).
Due to their central function as regulators of β-catenin, generally all proteins of the APC/Axin/GSK-3β complex would qualify as tumor suppressor genes. But, however, in contrast to APC and Axin, so far no mutations or deletions in the GSK-3β gene have been reported [58]. This might be explained by the fact that GSK-3β also phosphorylates other key regulatory proteins outside the Wnt pathway, such as proteins in insulin and growth factor signaling pathways [59]. Direct inhibition of GSK-3β function with the consequence of cellular accumulation of β-catenin has also been reported for the proteins Frat-1 [60], Akt/protein kinase B (PKB) [61], the βII isoform of protein kinase C (PKC-βII) [62], and polycystin-1 [63]. The proto-oncogene Akt/PKB is an extensively studied downstream target of insulin-like growth factor, integrin-linked kinase, and PI3-kinase signaling [64, 65]. Involvement of Akt/PKB in gastrointestinal carcinogenesis seems likely since insulin-like growth factor receptor has been found to be overexpressed in colorectal cancers [66]. PKC-βII has also been shown to be upregulated in colorectal cancer [67]and intracellular PKC-βII levels are increased after exposure to secondary bile acids, which are thought to be carcinogenic to the colonic epithelium [68].
APC: Gatekeeper of Colorectal Tumorigenesis
The APC gene was identified on chromosome 5q by genetic analysis of familial adenomatous polyposis (FAP) families [69, 70, 71]. In their early adulthood patients with FAP develop multiple adenomatous polyps of the colorectal epithelium, some of which progress to invasive carcinomas. Some FAP patients also suffer from extracolonic tumors, such as desmoid tumors, ampullary carcinomas, and hepatoblastomas [72, 73]. The sequence of the APC gene spans 15 exons and encodes a 2,843-amino acid protein of 310 kD. Subsequent studies defined the critical role of APC in the genesis of inherited and sporadic colorectal cancer and its main function as a regulator of free β-catenin [74, 75, 76]. While germline inactivation of APC mutations occurs throughout the entire gene, somatic mutations are clustered at the 5′ end of exon 15 between codons 1280 and 1500 (mutation cluster region, MCR; fig. 2b) [77]resulting in a frame shift or a premature stop codon and a truncated APC protein. Biallelic inactivation of APC usually results from a truncating mutation coupled with a deletion of the long arm of chromosome 5 [72]. Altogether, in 70–80% of all colorectal cancers APC function is inactivated by loss of APC expression or expression of a truncated protein [72].
The APC protein consists of multiple functional domains that mediate oligomerization and interaction with many cellular proteins including β-catenin [76, 78], γ-catenin [79, 80, 81], GSK-3β [49], Axin/conductin [51, 52, 53], tubulin [82, 83], EB1 [84], hDLG [85], Asef [86], and Siah-1 [87, 88]. However, the main function of APC seems to be the regulation of the free non-membrane-bound pool of β-catenin in concert with GSK-3β and the scaffold protein Axin/conductin. Truncated APC proteins loose their ability to bind to Axin which results in the inability to downregulate β-catenin, which then in turn accumulates in the cytoplasm and the nucleus [54, 89]. However, it has recently been shown that APC is also involved in the downregulation of non-phosphorylated, oncogenic forms of β-catenin which escape the aforementioned β-TrCP-dependent destruction [87, 88]. For this alternative pathway of destruction a different F-box protein, Ebi, is recruited. This alternative pathway requires the interaction of APC with Siah-1, a p53-inducible gene, which is also involved in the regulation of the tumor-suppressor gene, DCC [90]. As Siah-1 binds to the carboxy terminus of APC and most colorectal cancers carry truncating mutations lacking the carboxy terminus, both the Axin/GSK-3β/β-TrCP and the Siah-1/Ebi destruction pathways are abrogated. Consequently, only in tumors carrying β-catenin mutations and expressing wild-type APC, the Siah-1/Ebi system can have a functional role in regulating β-catenin.
As loss of APC function and oncogenic activation of β-catenin seem to be equally potent in terms of Tcf-transcriptional activation [91], it is surprising that APC mutations are found in the vast majority of colorectal cancers, whereas β-catenin mutations are only found in a subset of colorectal cancers wild-type for APC [58, 92, 93, 94, 95, 96]. This is particularly curious in light of the fact that both APC alleles need to be mutated versus only one β-catenin allele in order to deregulate Tcf signaling. One hypothesis to account for the highly discordant frequencies of APC and β-catenin mutations is that APC loss may provide the cell with a stronger growth advantage than activation of β-catenin, implying that APC has other vital functions besides promoting β-catenin degradation. Interestingly, β-catenin mutations are more frequent in small than in invasive adenomas, and tumors carrying mutated β-catenin are less aggressive than tumors showing loss of APC [96]. Of note, biallelic inactivation of APC outside colorectal tumors has only been reported for desmoid tumors [97]. These benign soft tissue tumors occur with an increased incidence in FAP patients. Up to 50% of sporadic desmoid tumors which are wild-type for APC carry β-catenin mutations [98, 99].
In addition to regulation of free β-catenin, APC has also been shown to have Wnt-independent functions mediated through its carboxy terminus, the region commonly lost in colorectal cancer. APC directly associates with the microtubule cytoskeleton and binds to microtubule-associated proteins of the EB/RP family [82, 83, 84, 100, 101]. Most recently APC has also been suggested to be involved in the maintenance of chromosomal stability through localization to the kinetochore of metaphase chromosomes, a function most likely dependent on the interaction with EB1 [14, 102, 103].
β-Catenin: Central Oncogene of the Wnt Pathway
β-Catenin and its close relative γ-catenin are the vertebrate homologs of the Drosophila gene armadillo [104, 105]. β-Catenin was first identified because of its binding to the cytoplasmic domain of the cell–cell adhesion protein E-cadherin [106, 107]. Under physiological conditions most cellular β-catenin is bound to E-cadherin, a process regulated by tyrosine kinases and tyrosine phosphatases [108]. Promotion of tyrosine phosphorylation of β-catenin by treatment of cells with epidermal growth factor or hepatocyte growth factor [109, 110]leads to its dissociation from the adherens junctions and to its transfer to the cytosol. Whether the phosphorylation is performed by the growth factor receptors themselves or by soluble tyrosine kinases is unknown. However, it has been shown in vitro that the hepatocyte growth factor receptor c-Met and the epidermal growth factor receptor c-erbB-2 bind to β-catenin [111, 112]. As described above, in the absence of a Wnt signal free β-catenin is then subsequently phosphorylated and degraded.
More recently, β-catenin has been implicated in human cancer [113]and its oncogenic potential has been extensively studied in in vitro tissue culture models [114, 115]and in vivoanimal models [116, 117, 118, 119]. Three separate mechanisms have been found to lead to accumulation of β-catenin in the cytoplasm and nucleus of cancer cells: inactivation of the APC tumor suppressor gene in colorectal cancer; Axin mutations in subsets of hepatocellular and colorectal cancers [56, 57], and mutations of β-catenin’s amino terminus in a variety of cancers (fig. 2c, 3). In as many as 50% of colon tumors with intact APC gainof-function mutations in the β-catenin gene have been identified [58, 91, 92, 93, 94]. The highest frequencies of β-catenin mutations in colorectal tumors have been found in the presence of microsatellite instability [94, 120]. It is important to note that inactivating of APC mutations and activating of β-catenin mutations never seem to coexist in a tumor.
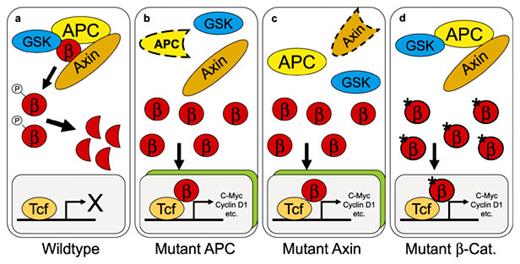
Deregulation of Wnt signaling in gastrointestinal tumors. a Under physiological conditions free β-catenin (β) is rapidly degraded. Three different mechanisms can lead to deregulation of Wnt/β-catenin/Tcf signaling. Inactivation of the tumor suppressor APC (b) or the scaffold protein Axin (c), and activating mutations of β-catenin itself (d) result in cellular accumulation of β-catenin. After nuclear translocation and binding to Tcf, transcription of specific target genes is activated.
Deregulation of Wnt signaling in gastrointestinal tumors. a Under physiological conditions free β-catenin (β) is rapidly degraded. Three different mechanisms can lead to deregulation of Wnt/β-catenin/Tcf signaling. Inactivation of the tumor suppressor APC (b) or the scaffold protein Axin (c), and activating mutations of β-catenin itself (d) result in cellular accumulation of β-catenin. After nuclear translocation and binding to Tcf, transcription of specific target genes is activated.
Besides colorectal cancers, liver malignancies are the gastrointestinal tumors with the second highest incidence of mutations in the Wnt pathway. Hepatoblastoma, the most common primary malignant liver neoplasm in childhood, occurring more frequently in FAP patients than in the general population, is probably the human tumor harboring the highest rate of β-catenin mutations with reported frequencies ranging from 52 up to 89% [121, 122, 123]. In HCC Wnt signaling can be activated through either β-catenin [124, 125, 126, 127]or Axin mutations [56]. The rate of β-catenin mutations in HCC has been reported to be about 20% and higher. In HCCs associated with hepatitis C the rate of β-catenin mutations may even exceed 40% [128].
In esophageal cancers so far no β-catenin, APC or Axin mutations have been reported [129, 130]. But, as mentioned above, overexpression of FzE3 in squamous cell esophageal cancers correlates with nuclear translocation of β-catenin [27]. Nuclear accumulation of β-catenin has also been observed in Barrett’s esophagus, a precursor of adenocarcinoma of the distal esophagus [131, 132, 133], suggesting an involvement of Wnt signaling in the genesis of both adenocarcinomas and squamous cell carcinomas of the esophagus. In intestinal-type gastric cancer β-catenin mutations have been reported to occur in 7 of 26 cases analyzed, but no β-catenin mutations were found in diffuse-type gastric cancer [134]. In 29 of 45 sporadic fundic gland polyps activating β-catenin mutations have been reported, whereas fundic gland polyps associated with FAP harbor germline APC mutations [135].
β-Catenin seems to play a minor role in the genesis of tumors of the pancreas, gallbladder, and biliary tract. So far no mutations of the β-catenin gene have been reported in pancreatic ductal cancer [136, 137], except for two mutations in pancreatic cancer cell lines [136]. However, just recently it was reported that three rare entities of pancreatic neoplasms, pancreatoblastomas, acinar cell carcinomas, and solid pseudopapillary tumors, frequently carry mutations in the β-catenin or APC genes [138, 139, 140]. In gallbladder adenomas β-catenin mutations have been described in about 60% of the cases analyzed, but interestingly β-catenin mutations are rare or absent in carcinomas or dysplasias of the gallbladder [141, 142]. No β-catenin mutations have been reported for intrahepatic cholangiocarcinoma [143]. In a study on biliary tract cancers only 8 of 107 cases had a β-catenin mutation [144].
In addition to gastrointestinal tumors, β-catenin mutations also occur with varying frequencies in gynecological tumors such as endometrial carcinoma [94, 145]and endometrioid ovarian carcinoma [146, 147, 148], in neoplasias of the skin such as melanoma [149]and pilomatricoma (with 75% of tumors harboring β-catenin mutations) [119], anaplastic thyroid carcinoma [150], prostate cancer [151], Wilms’ tumor [152], lung cancer [153], and medulloblastoma [154].
γ-Catenin is structurally and functionally a close relative of β-catenin. Both proteins bind to E-cadherin in adherens junctions, but only γ-catenin is also present in desmosomes [155]. Like β-catenin, γ-catenin also binds to APC [81, 156], Axin/conductin [157], and Tcf/Lef factors [158], carries a carboxy terminal transcriptional activation domain [159, 160], is regulated by APC [160], and its deregulated expression results in neoplastic transformation [160]. However, so far no γ-catenin mutations have been described in human cancers, except in one gastric cancer cell line [136].
The Final Step in Wnt Signaling: Activation of Tcf-Dependent Transcription
Cytosolic accumulation of β-catenin leads to the formation of complexes with Tcf/Lef transcription factors [161, 162]. After nuclear translocation of these complexes, Tcf/Lef factors facilitate gene-specific DNA binding while β-catenin serves as transcriptional activator. The histone acetyltransferases p300/CBP have been found to serve as transcriptional coactivators of Tcf/Lef target gene expression through interaction with β-catenin [163, 164, 165]. Moreover, Brg-1, a component of the mammalian SWI/SNF chromatin-remodelling complex, was also found to be indispensable for β-catenin/Tcf-target gene expression [166]. By binding of p300/CBP and Brg-1 to β-catenin’s carboxy terminus, the chromatin of regulatory regions of specific target gene promoters is remodeled facilitating the binding of the transcriptional machinery. The TATA-binding protein, Pontin52, is thought to mediate the contact between the β-catenin/Tcf-4 complex and the basal transcriptional machinery [167].
Of all Tcf isoforms Tcf-4 is the only Tcf protein being consistently expressed in colorectal epithelial cells [168]. Tcf-4 itself has been reported to carry mutations in a subset of colorectal cancers [169, 170, 171]. But, as all of these primary cancers and cancer cell lines also carry either inactivating APC or activating β-catenin mutations, these mutations of the Tcf-4 gene are not thought to substitute for APC or β-catenin mutations. A subset of the mutations affects the carboxy terminal region of Tcf-4 required for binding to CtBP, a member of the Groucho family of transcriptional repressors [172]. These repressors bind to Tcf-4 in the absence of β-catenin and hereby tightly control Tcf-4 activity [173, 174]. Therefore, Tcf-4 mutations are thought to have an additive rather than an initiating effect on neoplastic transformation. Once transformation of the colorectal epithelium has occurred, expression of Lef-1, a close homolog of Tcf-4, is upregulated. Only expression of the β-catenin-sensitive isoform of Lef-1 is upregulated and can support the activation of Tcf/Lef target genes [175].
Several β-catenin/Tcf target genes are supposed to contribute to tumor initiation and progression in mice and humans. Identification of c-Myc and cyclin D1 as β-catenin-regulated genes solved several longstanding puzzles. c-Myc is a proto-oncogene that has long been known to be overexpressed at the mRNA and protein levels in colorectal tumors [176, 177, 178, 179, 180]. But, however, the reason for c-Myc overexpression in colon tumors remained unknown. Unlike many other tumors c-Myc gene rearrangements or amplifications are rare in colon tumors [181]. The identification of c-Myc as a Tcf-4 target gene linked deregulated expression of the proto-oncogene c-Myc to APC and β-catenin [6]. Unlike in most other human tumor types, genetic alterations in the p16INK4a growth-inhibitory pathway, which includes Rb, cdk4, and cyclin D1, were only rarely found in colon tumors [182, 183, 184, 185]. The discovery of cyclin D1 as a target of the β-catenin/Tcf pathway also linked this pathway to colorectal carcinogenesis [7, 8].
Matrilysin/MMP-7 is another target gene with supposedly critical functions in cancer promotion [9, 186]. In the absence of the metalloproteinase MMP-7, intestinal tumorigenesis is strongly suppressed in APC mutant mice [187]. WISP-1 is a β-catenin/Tcf-4 target gene belonging to the CCN family of growth factors [188]and cells overexpressing WISP-1 reveal characteristics of transformed cells including induction of tumor growth in nude mice. Deregulated expression of the Tcf target gene gastrin in APC–/+ mice leads to an increase in polyp number, while gastrin-deficient APC–/+ mice exhibit a reduction in intestinal polyp formation [10]. The basic helix-loop-helix transcription factor ITF-2 is expressed in two different splice variants ITF-2A and ITF-2B. The expression of the longer protein ITF-2B was found to be directly regulated by β-catenin/Tcf and, when overexpressed, to induce transformation of epithelial cells [11].
Tcf-1 [189]and Axin-2/conductin [190, 191, 192, 193]have been identified as β-catenin/Tcf-4-regulated genes which negatively regulate Tcf signaling. Certain splice forms of Tcf-1 act as naturally occurring dominant-negative Tcf/Lefs which directly interfere with transcription of β-catenin/Tcf-target genes by specifically binding to Tcf sites in gene-regulatory regions. These proteins lack the amino terminal-binding domain for β-catenin, but still bind to DNA. The access for functional β-catenin/Tcf complexes to responsive promoters is therefore blocked. In vivo studies have found that mutational inactivation of Tcf-1 leads to formation of multiple adenomas in the organs of mice including the gut. Loss of Tcf-1 expression in mice heterozygous for APC enhances the growth of intestinal adenomas. Tcf-1 therefore seems to exert a tumor-suppressive function [172]. Axin-2/conductin, but not the homologous protein Axin, is induced by β-catenin. Via a negative feedback loop activated β-catenin/Tcf-4 signaling is downregulated by targeting β-catenin for degradation. Other genes proposed as targets of β-catenin/Tcf include the gap junction protein connexin 43 [194], the inhibitory basic helix-loop-helix factor Id2 [195], peroxisome proliferator-activated receptor-δ [196], survivin [197], as well as the genes c-jun, fra-1, uPAR, ZO-1 [198], NBL-4 [199], DRCTNNB1A [200], MDR1 [201], and brachyury [202].
Clinical Implications of Basic Research
Much interest focuses on the Wnt/APC/catenin/Tcf signal-transduction cascade. So far this research has yielded little clinical impact. However, based on the knowledge that most APC gene mutations result in a truncated protein, a so-called protein truncation test has been developed [203, 204]. This test, based on the in vitro transcription and translation of genomic PCR products of the APC gene, is used to prescreen FAP patients and their family members at risk. In case of a truncating mutation the detected protein is smaller in size than the corresponding wild-type product. By direct sequencing of the mutated DNA fragment APC mutations can be verified. As deaths from colorectal cancer can be avoided by early detection of colorectal adenomas and localized tumor stages much work has been invested into the development of DNA-based stool tests. Based on the large proportion of colorectal cancers with deregulation of Wnt signaling and the involvement of loss of APC function in very early steps of colorectal carcinogenesis, recently two molecular approaches to screen for colorectal cancer have been presented [205, 206]. In both studies APC mutations were reliably detected by either DNA sequencing or a digital protein truncation test. Future studies will have to show how useful these tests finally are to screen for colorectal cancer. But searching for APC mutations only will probably not be sufficient as the only marker indicating a colorectal neoplasm.
A correlation of β-catenin expression and cellular localization with the prognosis of several gastrointestinal tumors has been described. It has been found that strong nuclear or cytoplasmic β-catenin staining in colorectal cancer correlates with more invasive tumor growth, a higher susceptibility of disease recurrence after surgery, and a lower survival rate [207, 208, 209]. In HCCs, however, analysis of many cases revealed that mutation and nuclear staining of β-catenin correlated with less aggressive tumor growth and better survival rates [210, 211]. In contrast, in gastric cancers no correlation of β-catenin nuclear staining with tumor differentiation, tumor type, invasiveness or survival was found [212]. In esophageal carcinoma loss of proteins of the E-cadherin/β-catenin adhesive complex correlated with poor prognosis [213, 214].
In contrast to diagnostic and prognostic applications therapeutic approaches targeting the Wnt/APC/catenin pathway are even farther from clinical practice. Downregulation of β-catenin in order to inhibit β-catenin/Tcf signaling by adenovirus-based or antisense-based approaches has been shown to be critical for tumor cell growth in in vitro and in vivo models [215, 216]. Instead of downregulating β-catenin two approaches utilized synthetic Tcf-responsive promoters to drive expression of the apoptosis-inducing gene FADD or an enzyme catalyzing the activation of a cytotoxic prodrug, and both resulted in the selective death of colon cancer cells [217, 218]. However, all approaches targeting tumor cells with deregulated Tcf transcription are hampered by the fact that β-catenin/Tcf signaling also plays a critical role in tissue homeostasis as known from knock-out experiments with mice carrying a homozygous deletion of the Tcf-4 gene. These mice are incapable of maintaining a proliferative stem cell compartment in the small intestine and die shortly after birth [219]. Many questions remain unanswered, but undoubtedly the Wnt/APC/catenin pathway harbors great potential for future applications in cancer diagnosis, staging, and therapy.