Abstract
Survival analyses have been used to overcome some of the limitations encountered with other statistical analyses. Although extended Cox hazard modeling with time-dependent variables has been utilized in several medical studies, it has never been utilized in assessing the complex relationship between mutans streptococci (MS) acquisition (time-dependent covariate) and time to having dental caries (outcome). This study involved secondary analyses of data from a prospective study conducted at the University of Alabama at Birmingham. Low socioeconomic status, African-American preschool children from Perry County, AL, USA (n = 95) had dental examinations at age 1 year and annually thereafter until age 6 years by three calibrated dentists. Salivary MS tests were done at ages 1, 1.5, 2, 2.5, 3, and 4 years. The patterns of and relationship between initial MS detection (time-dependent covariate) and dental caries experience occurrence were assessed, using extended Cox hazard modeling. The median time without MS acquisition (50% of the children not having positive MS test) was 2 years. Approximately 79% of the children had positive salivary MS tests by the age of 4 years. The median caries experience survival (50% of the children not having dental caries) was 4 years. During the follow-up period, 65 of the children (68.4%) had their initial primary caries experience. Results of the extended Cox hazard modeling showed a significant overall/global relationship between initial caries experience event at any given time during the follow-up period and having a positive salivary MS test at any time during the follow-up period (hazard ratio = 2.25, 95% CI 1.06-4.75). In conclusion, the extended Cox modeling was used for the first time and its results showed a significant global/overall relationship between MS acquisition and dental caries. Further research using causal mediation analysis with survival data is necessary, where the mediator “presence of MS” is treated as a time-dependent variable.
It is now well established that mutans streptococci (MS) are the bacteria most commonly associated with the initiation of dental caries [Tanzer et al., 2001]. Of the MS found in the human oral cavity, Streptococcus mutans (Sm) usually predominates, while Streptococcus sobrinus (Ss) represents a minor fraction. Most children acquire MS soon after eruption of the primary teeth [Tanner et al., 2011; Gross et al., 2012]. Several studies have shown that the timing of the initial detection of MS varies between 7 and 36 months, and all studies linked MS detection to the time period coinciding with eruption of the primary teeth [Carlsson et al., 1975; Alaluusua and Renkonen, 1983; Fujiwara et al., 1991; Xu et al., 2014].
Previous longitudinal studies have assessed risk factors for dental caries experience in preschool children. Using an area-under-the-curve (AUC) composite, Ghazal et al. [2015] previously assessed risk factors for dental caries experience longitudinally in this study's cohort and found that greater frequency of toothbrushing and greater daily frequency of consumption of 100% juices were associated with lower incidence of dental caries (p = 0.01 and 0.049, OR = 0.34 and 0.37, respectively). Greater daily frequency of consumption of sweetened foods (AUC composite) was associated with greater incidence of early childhood caries (OR = 9.22, p = 0.002). Meurman and Pienihäkkinen [2010] found that the presence of MS, night feeding (yes vs. no), adding sugar to the diet (yes vs. no), and drinking of beverages other than water (yes vs. no) at age 18 months were significantly associated with caries incidence at age 42 months in 545 Finnish children.
Laitala et al. [2012] conducted survival analyses showing that MS-positive Finnish children (MS detected in their saliva at age 2 years, n = 29) had shorter time to having caries in both their primary and permanent teeth during the 10-year follow-up period compared to MS-negative children (no MS detected in their saliva at age 2 years, n = 119). The 50th percentile of dental caries survival (no caries in primary or permanent teeth) was 4.6 years in MS-positive children versus 8 years for MS-negative children (p = 0.001). At the end of their study, survival (no caries experience) was 10 and 36% in the MS-positive and MS-negative groups, respectively.
In other longitudinal studies, Köhler et al. [1988] found that 89% of the 78 Swedish children who had positive MS tests at age <2 years had caries at age 4 years (mean dfs score of 5). For children who had initial MS detection from ages 2 to 2.99, 3 to 3.99, and 4 to 4.99 years, 74, 36, and 25% had caries at age 4 years, respectively (mean dfs scores were 2.5, 0.9, and 0.3, respectively). Children with earlier initial MS detection had significantly greater caries experience (both any caries experience and dfs count). Alaluusua and Renkonen [1983] reported that Sm was detected in 13, 31, and 33% of 39 Finnish children who were examined at baseline at age 2, and followed up at ages 3 and 4 years. For the total 2-year follow-up period, 38% of the children had Sm detected and 41% had dental caries experience. Children who had Sm detected at age 2 had a significantly greater mean dmfs score (10.6) at age 4 compared to children who had Sm detected later (mean dmfs = 3.4, p < 0.005) or remained free from Sm infection (mean dmfs = 0.3, p < 0.0003). Also, Isokangas et al. [2000] found that the proportions of 195 Finnish children without caries experience at the annual examinations done at ages 2, 3, 4, and 5 years were greater in children who were in the MS-negative group at age 2 years. Thibodeau and O'Sullivan [1999] stated that dmfs scores in 85 children (mostly African-American and Hispanic from Hartford, CT, USA) were greater among those with >50 colony forming unit (CFU) at ages 4 and 5 (p < 0.05) compared to children with <50 CFU.
Some studies have not shown a significant relationship between MS detection and dental caries. For example, Matsuda et al. [1979] assessed the relationship between Sm detection and caries incidence (yes/no) of the maxillary primary incisors during a 30-month follow-up. No carious lesion was found before Sm was detected in 22 Japanese infants who were 5-13 months old. However, the results did not show a statistically significant relationship between dental caries incidence and previous detection of Sm (p > 0.05).
Although there are several studies which assessed the relationship between the presence of MS (yes/no or CFU count) as an independent covariate and dental caries as an outcome, the presence of MS was assessed cross-sectionally at a specific time point (although caries outcomes might have been assessed longitudinally). In other words, children who were included in the analysis were defined either as having MS or not at baseline. Previous studies did not address the fact that the MS variable is really a “moving target.” During child development, not only do dietary and oral hygiene factors change, but the patterns of MS detection also change. Children can have negative salivary MS tests at one study visit and positive salivary MS tests at another study visit. This current study addressed this critical issue and dealt with the MS variable as a time-dependent variable, using extended Cox hazard modeling in the statistical analysis.
This is the first study to use this analytic approach with the purpose of assessing the global/overall relationship between MS detection and dental caries experience, which has never been reported in the dental literature.
Methods
This report involves secondary analyses of data from a longitudinal study conducted at the University of Alabama at Birmingham (UAB) [Ghazal et al., 2015]. A cohort of low socioeconomic status, African-American children was recruited in 2008. Inclusion criteria were that infant-aged children (mean age approx. 1 year) were required to live with their biological mother and have at least one biological sibling, and the mothers had to say that they planned to remain in the area for at least 3 years. Institutional Review Board approval was obtained from UAB and informed consent and waiver of assent were obtained from the caregivers. All children received fluoride varnish at the initial recruitment visit and then semi-annually at their follow-up visits.
Oral samples for MS data were obtained at average ages of 1, 1.5, 2, 2.5, 3, and 4 years. MS data at ages 5, and 6 years were not used in this analysis due to a change in the study protocol to PCR detection [Childers et al., 2017]. Oral samples were collected from each child for MS analysis using sterile tongue scraping or cotton swab at all study visits.
Dental examinations were conducted by three calibrated dentists when children were at average ages of 1, 2, 3, 4, 5 and 6 years. A total of 23 children aged 3 or 4 years were examined to assess inter- and intraexaminer reliability. Dental caries was reported at the cavitated level following World Health Organization (WHO) criteria [WHO, 1987].
For the purpose of data analyses, birth was considered the baseline and all children were assumed to have negative salivary MS tests then. Kaplan-Meier survival curves were plotted for the preschool children to demonstrate the patterns of both (1) initial MS detection and (2) dental caries occurrence. Also, salivary MS patterns were assessed at the individual level as the presence/absence of salivary MS at baseline (birth) and at the study visits at age 1, 1.5, 2, 2.5, 3, and 4 years.
The bivariate relationship between MS detection (time-dependent covariate) and time to having dental caries experience was assessed using the extended Cox hazard modeling. The dataset was restructured as follows (summarized in Table 1). First, for children who had negative salivary MS tests at all study visits or a positive salivary MS test at the same time as caries experience diagnosis or censoring, one observation was created for each of these children quantifying the time interval from the beginning of the study until caries experience diagnosis or censoring (lost to follow-up or end of the study follow-up). During this interval, these children were marked as having a negative salivary MS test, while the caries event was marked as either (a) yes, if caries experience was diagnosed before the end of the study or (b) no, if censoring occurred before caries experience was ever diagnosed.
Summary of how the dataset was restructured and how mutans streptococci (MS) event and caries status were defined before the extended Cox hazard modeling was used

Second, for children who had positive salivary MS tests before caries experience diagnosis or censoring (lost to follow-up or end of the study follow-up), two observations were created: (a) the first observation quantified the time interval from the beginning of the study until just before MS was detected (during this interval, these children were marked as having a negative salivary MS test, while caries event was marked as “no”), and (b) the second observation quantified the time interval from the time children had initial positive salivary MS test until caries experience diagnosis or censoring occurred (during this period, these individuals were marked as having a positive salivary MS test, while caries event was marked as (a) yes, if caries experience was diagnosed before the end of the study and (b) no, if censoring occurred before caries experience was ever diagnosed).
Third, for individuals who had the first positive salivary MS test after caries experience diagnosis, one observation was created to quantify the time interval from the beginning of the study until the time of caries experience diagnosis. During this period, these individuals were marked as having a negative salivary MS test while caries event was marked as “yes.”
After the dataset was restructured, hazard ratios (HR) and their corresponding 95% confidence intervals (95% CI) were determined to assess the bivariate relationship between MS detection (as a time-dependent variable) and time to having dental caries experience. This current study did not adjust for possible confounders because of the limited sample size.
For the purpose of comparison, the bivariate relationships between MS detection (yes/no) and dental caries experience (yes/no) were also assessed using traditional statistical methods. First, logistic regression was used to assess the differences in the prevalence of caries experience (yes/no) at each annual clinical examination separately in children with all negative salivary MS tests at or before the age of 1.5 years versus a positive salivary MS test by the age of 1.5 years. Second, logistic regression was used to assess the differences in the prevalence of caries experience (yes/no) at each annual clinical examination separately in children with negative salivary MS tests versus positive salivary MS tests at that exact study visit (cross-sectionally).
All data analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC, USA), using PROC LOGISTIC, PROC LIFETEST, and PROC PHREG. The significance level was set at p < 0.05.
Results
At approximately age 1 year, there were 95 children with available dental examination and salivary MS data (mean age 0.95 years, SD ±0.31). When comparing the results of the total dmfs count at the person level, the average intraexaminer weighted kappa statistic for 17 children aged 3-4 years was 0.91, and the average interexaminer weighted kappa for 23 children aged 3-4 years was 0.93 [Ghazal et al., 2014]. For MS detection, the positive predictive value was 0.94. Negative predictive value, sensitivity, and specificity were not assessed because it was beyond the scope of the parent study. Figure 1 shows that the 75th, 50th, and 25th MS survival percentiles (meaning no MS recorded) were 1.5, 2, and 2.5 years, respectively, and the mean survival time was 2.18 years (SD 0.10). Also, during the follow-up period, 75 of the children (78.9%) had at least one positive salivary MS test and 20 (21.1%) were censored. Figure 1 also shows that the 75th, 50th, and 25th caries survival percentiles were 3, 4, and 5 years, respectively, and the mean survival time was 3.95 years (SD 0.14). During the follow-up period, 65 of the children (68.4%) had caries experience and 30 (31.6%) were censored.
Kaplan-Meier survival curves for mutans streptococci (MS) initial detection (a) and permanent tooth caries experience (b). At birth (age = 0), all individuals were assumed to be MS negative and caries free. There were 95 children at baseline.
Kaplan-Meier survival curves for mutans streptococci (MS) initial detection (a) and permanent tooth caries experience (b). At birth (age = 0), all individuals were assumed to be MS negative and caries free. There were 95 children at baseline.
Table 2 shows the results of the bivariate survival analysis assessing the relationship between initial MS detection (time-dependent variable) and time to dental caries occurrence. Extended Cox hazard modeling showed that the overall hazard rate for a caries experience event at any given time during the follow-up period among children with a positive salivary MS test (at a point in time) was 2.25 times as great as that among children with a negative salivary MS test (at the same point in time) (HR = 2.25, 95% CI 1.06-4.75).
Bivariate analysis results assessing the relationship between mutans streptococci (MS) detection and time to dental caries experience (n = 95)

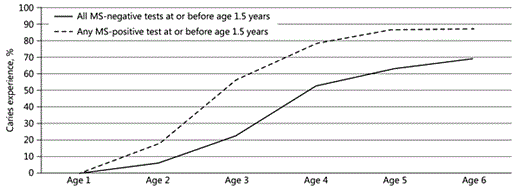
Additional analyses show caries prevalence (yes/no) at annual clinical examinations among children who had any positive salivary MS test at or before the age of 1.5 years versus all negative salivary MS tests at or before the age of 1.5 years (Fig. 2). The differences were statistically significant at ages 3 years (p = 0.002, OR = 4.40, 95% CI 1.71-11.35), 4 years (p = 0.020, OR = 3.28, 95% CI 1.21-8.91), and 5 years (p = 0.031, OR = 3.94, 95% CI 1.14-13.60). Figure 3 shows caries prevalence (yes/no) at annual clinical examinations among children who had a positive salivary MS test at that examination point versus those with negative salivary MS test at that examination point (3 cross-sectional tests, since there were no MS data after age 4). The difference was statistically significant only at age 2 (p = 0.048, OR = 5.13, 95% CI 1.08-25.82). The differences in caries prevalence among children who had negative salivary MS tests at age 1 versus positive salivary MS tests at age 1 were not statistically significant at any age (data not shown).
Percentage of subjects with caries experience at annual clinical examinations among children with any mutans streptococci (MS)-positive versus all MS-negative tests at or before the age of 1.5 years. Using logistic regression, differences were statistically significant at ages 3 (n = 83, p = 0.002, OR = 4.40, 95% CI 1.71-11.35), 4 (n = 77, p = 0.020, OR = 3.28, 95% CI 1.21-8.91), and 5 years (n = 69, p = 0.031, OR = 3.94, 95% CI 1.14-13.60).
Percentage of subjects with caries experience at annual clinical examinations among children with any mutans streptococci (MS)-positive versus all MS-negative tests at or before the age of 1.5 years. Using logistic regression, differences were statistically significant at ages 3 (n = 83, p = 0.002, OR = 4.40, 95% CI 1.71-11.35), 4 (n = 77, p = 0.020, OR = 3.28, 95% CI 1.21-8.91), and 5 years (n = 69, p = 0.031, OR = 3.94, 95% CI 1.14-13.60).
Percentage of subjects with caries experience at annual clinical examinations among children with mutans streptococci (MS)-positive versus MS-negative tests at that examination point. Using logistic regression, the difference was statistically significant only at age 2 (n = 83, p = 0.048, OR = 5.13, 95% CI 1.08-25.82). After age 3, there were more than 80% missing values in the MS data, so they were not included in the graph.
Percentage of subjects with caries experience at annual clinical examinations among children with mutans streptococci (MS)-positive versus MS-negative tests at that examination point. Using logistic regression, the difference was statistically significant only at age 2 (n = 83, p = 0.048, OR = 5.13, 95% CI 1.08-25.82). After age 3, there were more than 80% missing values in the MS data, so they were not included in the graph.
Discussion
Most children acquire MS bacteria in the oral cavity early in life after eruption of the primary teeth. MS have the cariogenic properties of sucrose metabolism and acid production through the process of fermentation [Banas, 2004]. Also, MS synthesize extracellular glucans that facilitate biofilm formation [Banas, 2004]. It has been stated that “MS are necessary for fissure caries development, but not sufficient” [Burt et al., 1983]. MS detection occurs in children with and without observable dental caries and is reported to be responsible for creating the retentive niche for the colonization of lactobacilli, and potentially other bacteria involved in this disease [Caufield et al., 2012, 2015].
Several studies have assessed the relationship between the presence of MS (yes/no or CFU count) and dental caries. However, no previous study assessed MS detection as a time-dependent covariate to capture changes in the pattern of MS detection over the follow-up period.
Although this study did not identify new biological relationships, this was the first caries study in which the hazard for having dental caries was assessed in the following way: at each time (t) when child (x) experienced dental caries, comparisons were made with the current MS status for child (x) in relation to the current MS values of all other children who were at risk of having dental caries at time (t). Accounting for the time-varying nature of MS detection can provide useful insights regarding some specific time patterns of both MS detection and caries occurrence and valuable biological information that could be missed otherwise. For example, if the traditional Cox proportional hazard modeling had been used to assess the relationship between MS acquisition (yes/no) and dental caries experience occurrence, the only MS data that would have been used were the baseline MS data at age 1 year, since with Cox proportional hazard modeling, the predictor variables must be assessed prior to the survival outcome. Thus, substantial valuable information could have been lost if extended Cox hazard modeling had not been used.
Practical issues surrounding the implementation of the extended Cox hazard modeling are quite complex. In this regard, a logistical challenge in the analysis reported herein was the data management and recoding necessary to describe the values of time-dependent covariates in the manner required by the SAS software. Results of extended Cox hazard modeling showed a significant relationship between having a caries experience event at any given time during the follow-up period and having a positive salivary MS test at the same time point (HR = 2.25, 95% CI 1.06-4.75).
Extended Cox hazard modeling with time-dependent covariates is a powerful tool for exploring predictive relationships by assessing risk and preventive factors that can vary over time. The careful implementation of extended Cox hazard modeling can prevent (1) misleading effect estimates and (2) missing major effects in the early or late follow-up period, which can happen when using the traditional survival analysis.
With extended Cox hazard modeling, the HR for caries associated with MS bacteria requires that the comparison be made at the same time. Hence, the HR is valid only for those time points at which some children had and some did not have salivary MS detected. In the model, the comparison group at any time (t) with MS = 0 includes both subjects who never had MS detected and those who had MS detected after time (t).
Additional analyses conducted in this study showed that the association between the presence of MS and caries prevalence (yes/no) was not statistically significant at all study visits. For example, the presence of any positive salivary MS test at or before the age of 1.5 years was significantly associated with caries (yes/no) at ages 3, 4, and 5 years, but not at ages 2 or 6 years. This illustrates that it is easy to miss the significant relationships between caries experience and the presence of MS if a less favorable study question and/or time periods were used. This shows the importance of the use of extended Cox hazard modeling, as it provided a global/overall assessment of the effect of assessed risk factors on time to having dental caries. Thus, what was unique in the interpretation was that it was not time specific.
A major strength of this study is the implementation of the extended Cox hazard modeling, which used MS detection as a time-dependent variable and incorporation of the “time” factor into the caries outcome. The unique contribution to the science is in the interpretation of the findings. Instead of saying that MS detection at age 2, per se, was associated with experiencing dental caries earlier in life, we say the overall MS detection (global assessment throughout the follow-up period) was associated with experiencing dental caries earlier in life. Other strengths included (1) the homogenous and relatively stable study sample of African-American children with regards to socioeconomic status, educational level, and fluoride exposure and (2) the narrow bands of children's ages.
The main limitations of this study were (1) limited sample size, (2) possible underestimation of the outcome due to lack of radiographs, (3) the lack of adjustment for other potential confounders, and (4) the inability to generalize the study findings to other populations.
The understanding about the role of MS in tooth decay remains incomplete. In addition to major gaps in the knowledge concerning the cultural and genetic bases for MS acquisition, gaps remain regarding the most appropriate analytic techniques for assessing the relationship between MS acquisition and dental caries.
Acknowledgments
The National Institute of Dental and Craniofacial Research (grant: R01-DE016684) supported this study. The authors would like to thank the dentists (examiners), Pediatric Dentistry residents, clinical coordinators, and clinical and laboratory personnel who contributed to this study.
Disclosure Statement
There are no financial or other relationships that lead to a conflict of interest.
Author Contributions
Author contributions included participation in study design (T.S.G., S.M.L., N.K.C., K.D.C., D.J.C., J.J.W., J.E.C., and J.K.), data acquisition (N.K.C.), and data analysis and interpretation (T.S.G., S.M.L., K.D.C., and J.E.C.).