Abstract
Background: In patients with chronic lung diseases, the work rate for endurance training is calculated by the maximal work rate (Wmax). Because the assessment bears side effects, a prediction by easier accessible tests would be of practical use. Objective: We addressed the reliability of predicting Wmax on the basis of the 6-min walk distance (6MWD) test and a set of further parameters in patients with different lung diseases. Methods: Baseline data of a longitudinal study including 6MWD, Wmax, peripheral muscle force, lung function, fat-free mass and dyspnea (Modified Medical Research Council score) of 255 men with occupational lung diseases (104 asthma, 69 asbestosis, 42 silicosis, 40 chronic obstructive pulmonary disease) were evaluated. Results: 6MWD correlated with Wmax (r = 0.51, p < 0.05). The product of 6MWD and body weight, in particular fat-free mass, led to an improvement in the correlation of Wmax with 6MWD. Muscle force, lung function and Modified Medical Research Council score correlated moderately but significantly with Wmax (p < 0.05 each). The maximum correlation gained by including 6MWD and further parameters in the prediction equations was r = 0.76 in patients with obstructive lung function impairment and r = 0.61 in asbestosis patients. The residual standard deviations of Wmax predicted by the calculated equations ranged between 20 and 28 W, and the 95% prediction intervals of Wmax ranged between ±47 and ±65 W. Conclusions: A reliable prediction of individual Wmax by 6MWD or related measures and therefore a replacement by other tests is not possible. Nevertheless, it may be useful for the comparison of average values in epidemiological and clinical studies.
Introduction
Exercise training is a recognized therapy option in patients with chronic respiratory diseases regardless of underlying causes [1,2]. Within rehabilitation programs [3], endurance training is usually conducted on cycle ergometers at a work rate of at least 60% of individual maximal work capacity [3]. The assessment of maximal work capacity requires special equipment and well-trained staff, is expensive, time consuming and bears the risk of side effects.
Therefore, a prediction of the maximal work rate (Wmax) by more easily assessed measures would be of practical relevance. Regarding patients with chronic obstructive pulmonary disease (COPD), equations for predicting Wmax from the 6-min walk distance (6MWD) test have been presented [4,5,6,7,8]. One study also offered an equation for patients with lung fibrosis [6]. Four of these studies were based on small samples. To evaluate the applicability of prediction, larger study groups with different respiratory diseases are needed.
Based on these considerations, we reanalyzed the baseline data from a large study on the efficacy of rehabilitation [9]. We aimed to quantify the correlations of Wmax with other measures and to assess the maximal reliability of individual prediction by including a broad range of predictors. For clarification, we also compared the results with existing prediction equations for Wmax.
Methods
Study Design and Subjects
Subjects were recruited within a longitudinal clinical study on the efficacy of pulmonary rehabilitation in 287 patients with occupational lung diseases. Baseline data before intervention were reanalyzed in a subpopulation of 255 men.
This study was approved by the German Social Accident Insurance according to official ethic regulations (project No. FFFB0094). Patients gave their informed consent.
The inclusion criteria were: recognized occupational respiratory disease diagnosed as asthma, asbestosis, silicosis or COPD in coal miners; functional impairment leading to a reduction in earning capacity by 20-50%, age <75 years; no rehabilitation in the previous 2 years; maximum exercise capacity of at least 40 W; no progressive malignant diseases; clinically stable.
Assessments included height, age, weight, body fat by near-infrared light measurement (Futrex 6100 XL, Futrex Inc., USA), spirometry (Master Screen Body, Care Fusion Germany), peripheral muscle force, 6MWD, Wmax and dyspnea at rest [Modified Medical Research Council (MMRC) questionnaire].
The 6MWD was determined 2 times on the first day (resting time of ≥40 min in between) and once on the following day according to American Thoracic Society guidelines [10]. Wmax was assessed by incremental cycle ergometry test (Master Screen CPX, Care Fusion Germany; e-Bike Basic PC Plus-Ctrl, Ergoline GmbH, Germany). After a resting period of 3 min while already sitting on the ergometer, patients started cycling at a work rate of 30 W. The work rate was increased every 2 min by 20 W until symptom limitation. Wmax was defined as the highest work rate achieved for at least 30 s.
Quadriceps muscle force was measured by the ‘DigiMax-Muskelfunktion-Testcenter' (DigiMax, MechaTronics, Hamm, Germany) and handgrip force by a hydraulic hand dynamometer (JAMAR, Lafayette Instrument, USA); the best value of 3 tests was taken. Results were expressed as the sum of both extremities in kilograms.
Statistical Analysis
For data description, absolute and relative frequencies as well as mean values, standard deviations (SDs) and ranges were computed. Subgroups were compared with each other using one-way analysis of variance (ANOVA) and χ2 tests of contingency tables. If overall differences turned out to be statistically significant, appropriate post hoc multiple comparisons were made using the Student-Newman-Keuls multiple range test. The total group of patients and the subgroups with asthma, asbestosis, silicosis or COPD, or the pooled data of asthma, silicosis and COPD were analyzed. The adequacy and admissibility of ANOVA was checked by standard procedures regarding data distributions and residuals.
Multiple stepwise linear regression analysis of Wmax was used to identify the predictors. To allow practical conclusions, the accuracy of prediction was expressed not only by the explained variance and Pearson correlation coefficients but also by the residual SD of the prediction and the 95% prediction intervals which refer to the prediction of the value of a newly recruited individual who was not part of the group used for analysis. The level of statistical significance was assumed as p = 0.05. All calculations were performed with the software SPSS 19.
Results
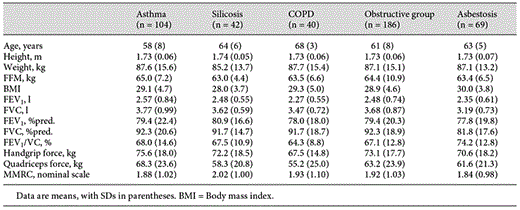
The present study is based on data of the baseline examination of the subgroup of men (n = 255): 104 with asthma, 42 with silicosis, 69 with asbestosis and 40 with COPD; the subgroup of women was excluded, being too small to allow statistically reliable conclusions. The study was conducted from March 2007 to May 2010. Characteristics of study subjects are given in table 1.
6MWD Test
The comparison of 6MWD values of the three tests on 2 days is shown in table 2.
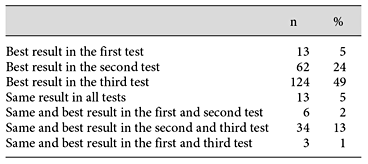
Upon statistical testing, the mean difference between the first and second test (mean ± SD, 16 ± 34 m) turned out to be significantly different from the difference between the second and third test (6 ± 42 m). We considered the first test as run-in test and selected the individual best test out of the second and third test.
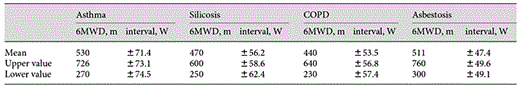
The results for asthma, silicosis, asbestosis and COPD are shown in table 2. 6MWD was shorter in the COPD and silicosis groups compared to the two other groups (ANOVA, p < 0.05 each). The mean 6MWD in the total group was 502 m (range 230-670).
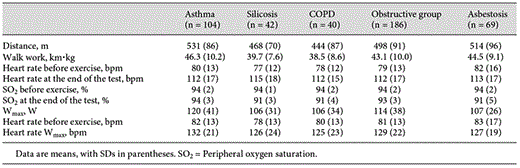
Maximal Work Capacity
ANOVA revealed significantly higher values in asthma patients (p < 0.05). Data are given in table 3. The mean maximal work capacity in the total population was 112 W (range 40-230).
Correlation between 6MWD and Wmax
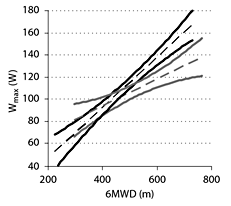
The total population showed a significant correlation (r = 0.51, p < 0.05) between 6MWD and Wmax. Figure 1 displays the relationships in the disease groups. When regression lines were tested for differences by pairwise comparisons, intercepts and slopes proved to be similar in the silicosis, asthma and COPD subgroups but different in the asbestosis subgroup (p < 0.05). Based on these results, silicosis, asthma and COPD were analyzed together called ‘obstructive group' (table 1). The asbestosis group was analyzed separately. Regression lines and their confidence intervals for both groups are shown in figure 2.
a-d Relationship between 6MWD and Wmax. Regression lines (solid lines) and their equations are given for the four disease groups, as well as 95% prediction intervals (dashed lines) for a newly included individual.
a-d Relationship between 6MWD and Wmax. Regression lines (solid lines) and their equations are given for the four disease groups, as well as 95% prediction intervals (dashed lines) for a newly included individual.
Confidence intervals of the regression lines of Wmax as function of 6MWD. Regression lines (dashed lines) and their 95% confidence intervals (solid lines) for Wmax as function of 6MWD in the obstructive group (black) and the asbestosis group (grey).
Confidence intervals of the regression lines of Wmax as function of 6MWD. Regression lines (dashed lines) and their 95% confidence intervals (solid lines) for Wmax as function of 6MWD in the obstructive group (black) and the asbestosis group (grey).
Correlation of Further Parameters with Wmax
Table 4 shows the single correlations between Wmax and additional parameters. To identify the best combinations of predictors, we defined the entities ‘walking test', ‘lung function' and ‘extremity muscle force'.
• Walking test: 6MWD, 6-min walk work (6MWW) calculated by multiplying 6MWD in km and body weight in kg, or 6-min walk fat-free mass (6MWFFM), the product of 6MWD in km and FFM in kg.
• Lung function: forced expiratory volume in 1 s (FEV1) in liters, forced vital capacity (FVC) in liters, their percent predicted values, or their ratio in percent (Tiffeneau quotient).
• Extremity muscle force: handgrip or quadriceps muscle force.
To deal with the colinearity, multiple linear regression was performed with all possible combinations of just one parameter from each above-mentioned entity to identify the best predictor.
Prediction Models for Wmax in Different Groups of Diseases
In the obstructive group, the maximal overall correlation reached r = 0.758. In the stepwise multivariate analysis, the anthropometric parameters turned out to become insignificant and were excluded, while 6MWFFM, FVC, MMRC and quadriceps muscle force remained significant (p < 0.05 each). Including these measures, the correlation was r = 0.748 and the prediction equation is:
(a) Wmax pred. = -6.117 + 2.491·6MWFFM (km·kg) - 5.926·MMRC + 10.118·FVC (l) + 0.232·quadriceps muscle force (kg).
Measurement of quadriceps muscle force is not routinely available. We excluded this predictor despite being statistically significant. The correlation was r = 0.737 corresponding to the equation:
(b) Wmax pred. = -1.786 + 2.793·6MWFFM (km·kg) - 6.723·MMRC + 10.748·FVC (l).
Values of FFM might not be available. The next best model was based on the 6MWW, with a correlation of r = 0.721 and the following equation:
(c) Wmax pred. = 9.177 + 1.621·6MWW (km·kg) - 8.121·MMRC + 13.768·FVC (l).
When substituting 6MWW by 6MWD, the correlation was reduced to r = 0.677:
(d) Wmax pred. = 2.888 + 0.140·6MWD (m) - 7.533·MMRC + 15.224·FVC (l).
In the asbestosis group, the maximal overall correlation reached r = 0.623. In the combined, stepwise model, quadriceps muscle force and body mass index were excluded, being insignificant, whereas 6MWD, FVC and MMRC remained significant (p < 0.05 each). The corresponding correlation was r = 0.607. The equation was as follows:
(e) Wmax pred. = 57.287 + 0.072·6MWD (m) - 9.456·MMRC + 9.676·FVC (l).
Residuals and Prediction Intervals
In the obstructive group, SDs of the residuals ranged between 25.678 for equation (a) and 28.108 for equation (d); in the asbestosis group, the SD was 20.859. We also computed 95% prediction intervals that describe the prediction for a newly included patient. Prediction intervals were calculated with 6MWD being the only independent variable in linear regression to ensure the comparability between regressions and the literature. Details concerning the single disease groups are given in table 5. The 95% prediction intervals are also graphically demonstrated in figure 1, in addition to the individual values and the regression line.
Comparison with Prediction Equations from the Literature
Figure 3a illustrates the residuals of predicted Wmax of the obstructive group in dependence of the 6MWD, using equation (a) as given above. For comparison, we applied the four equations for estimating Wmax as found in the literature [4,5,6,7] to the data of our population. The residuals of these calculations are shown in figure 3b-e. The same was done for the asbestosis group using equation (e) and the one equation from the literature [6] for estimating Wmax in lung fibrosis patients, as demonstrated in figure 4b.
a-f Residuals of predicted Wmax of COPD patients in comparison to residuals of other equations in the literature applied to the data set. a Residuals of data from this study according to equation (a) (see Results) in the obstructive group (n = 186) versus the 6MWD. b-f Residuals, having applied equations from references [4,5,6,7,8] to the data from this study for the obstructive group (n = 186) versus the 6MWD. b 103.217 + 30.500 (= male gender) + [-1.613·age (years) + (0.002·6MWW [km·kg])] [7]. c -51.994 - 0.505 (= male gender) - [0.234·age (years)] + [0.091·height (cm)] + [0.132·6MWD (m)] [8]. d 17.393 + [1.442·6MWW (km·kg)] [5]. e 2.310·6MWW (km·kg) + 8.820 [6]. f -27.9717 + [3.7792·6MWFFM (km·kg)] [4].
a-f Residuals of predicted Wmax of COPD patients in comparison to residuals of other equations in the literature applied to the data set. a Residuals of data from this study according to equation (a) (see Results) in the obstructive group (n = 186) versus the 6MWD. b-f Residuals, having applied equations from references [4,5,6,7,8] to the data from this study for the obstructive group (n = 186) versus the 6MWD. b 103.217 + 30.500 (= male gender) + [-1.613·age (years) + (0.002·6MWW [km·kg])] [7]. c -51.994 - 0.505 (= male gender) - [0.234·age (years)] + [0.091·height (cm)] + [0.132·6MWD (m)] [8]. d 17.393 + [1.442·6MWW (km·kg)] [5]. e 2.310·6MWW (km·kg) + 8.820 [6]. f -27.9717 + [3.7792·6MWFFM (km·kg)] [4].
a, b Residuals of predicted Wmax of asbestosis patients in comparison to residuals of the equation in the literature for idiopathic pulmonary fibrosis patients applied to the data set. a Residuals of the present data according to equation (e) (see Results) in the asbestosis group (n = 69) versus the 6MWD. b Residuals according to the equation from reference [6] (0.122·6MWD) + (0.387·FVC %pred.) - 21.474 applied to our own data, for the asbestosis group (n = 69), versus the 6MWD.
a, b Residuals of predicted Wmax of asbestosis patients in comparison to residuals of the equation in the literature for idiopathic pulmonary fibrosis patients applied to the data set. a Residuals of the present data according to equation (e) (see Results) in the asbestosis group (n = 69) versus the 6MWD. b Residuals according to the equation from reference [6] (0.122·6MWD) + (0.387·FVC %pred.) - 21.474 applied to our own data, for the asbestosis group (n = 69), versus the 6MWD.
Discussion
Training of exercise capacity is an important issue in the rehabilitation of patients with chronic lung diseases [11]. Commonly, the intensity of training is calculated by the maximal work capacity. The prediction of maximal work capacity by more easily assessed measures would be of practical value.
We found significant correlations between Wmax and 6MWD or related measures in a large population of patients with four different respiratory disorders in men (n = 255). The correlations were weaker than those reported in the literature [4,5,6,7,8]. This might be explained by our less homogeneous but, from a clinical perspective, more realistic patient cohort. We aimed to evaluate a broad panel of predictors of Wmax from a statistical point of view, but also to analyze subsets of predictors that are likely to be available in clinical practice.
In patients with obstructive airway diseases comprising asthma, COPD and silicosis, the correlations with Wmax were homogeneous and could be pooled. The product of 6MWD and FFM proved to be a better predictor than 6MWD itself or than walk work, i.e. the product of 6MWD and body weight. These findings are in accordance with published data [12]. Since COPD patients often exhibit reduced muscle mass relative to body weight, the FFM reflects the actual muscle mass better than body weight [4].
The prediction of Wmax was improved by including dyspnea score, FVC and quadriceps muscle force. The MMRC is a simple means for assessing the impact of breathlessness in daily life. It has been described to be correlated with FVC as well as with 6MWD [13].
Concerning lung function, FVC was the best predictor, independent of the lung disease. In restrictive disorders, vital capacity is directly affected, and thus, a correlation is plausible [14]. In COPD, the correlation can be explained by a reduced inspiratory capacity due to dynamic lung hyperinflation [15,16]. In advanced COPD, dynamic hyperinflation is observed not only in maximal exercise testing but also after the 6MWD [17,18].
In patients with obstructive lung disorders, quadriceps muscle force was an additional predictor of Wmax. Besides lung function impairment and dyspnea, peripheral muscle weakness contributes to exercise limitation in COPD [19]. Quadriceps muscle weakness could be shown to occur in about 25% of patients with COPD without correlation to disease severity [20]. Quadriceps muscle dysfunction can be found not only if FFM is reduced but also in some patients with preserved FFM [21].
Patients with asbestosis, resembling a restrictive lung disorder, showed a weaker relationship between Wmax and 6MWD compared to obstructive diseases. Inclusion of the FVC and MMRC improved the correlation.
Although we have included many predictors, even the optimal regression model explained only 57% of variance in the obstructive group, and in the asbestosis group <50%. Other unknown factors must have contributed to this result or the inherent variability of tests plays a role. Both 6MWD and Wmax address exercise tolerance, both depending on motivation. While 6MWD is sensitive in patients with advanced impairment, its value is limited in less impaired patients due to a ceiling effect. This might explain part of the variability in the relationship between both measures. Problems of coordination may also have a different impact.
The 6MWD is known to exhibit a learning effect [22,23]. In our data, the first 6MWD was significantly shorter, whereas the second and third test did not differ statistically. This confirms that one training test is necessary to gain reliable results. Eiser et al. [22] calculated an 8% intrasubject variation of 6MWD. In our population, this would correspond to a variation between about 18 and 58 m for the minimum and maximum 6MWD. This also limits the accuracy of prediction. A variation of 50 m in 6MWD would induce a variation of predicted Wmax of 11.6 W.
The reproducibility of Wmax is more difficult to assess as the test aims at exhaustion. Literature data suggest an intrasubject variability of about 10% in COPD [24,25,26]. In our study, a variability of 10% would correspond to variations between 4 and 23 W, and about 11.4 W on average. The variability of predicted Wmax as indicated by the residual SD was much larger. This confirms the assumption that the variability in the predictor 6MWD also has an impact on the reliability of the predicted Wmax. As a result, even in the optimal models, the 95% prediction intervals were wide and the maximally achievable accuracy of prediction in single individuals is low. Sillen et al. [8] came to a similar conclusion: even after calculating a regression equation in a COPD cohort of nearly 3,000 patients using 6MWD, anthropometric parameters and FEV1 as predictors, the estimation of Wmax was too inaccurate to be of practical value in an individual. Ross et al. [27] stated a comparable result for the estimation of peak oxygen uptake via 6MWD, with data indicating a residual standard error of peak VO2 as high as 26.7%.
For clarification and comparison, we also applied published prediction equations of Wmax to our data. Optional additional predictors in these studies were age, gender, body weight or FFM and FEV1. In the obstructive group, the residuals of two equations [7,8] showed similar variance and distribution to our equation, with gender being part of both equations. However, the other three equations [4,5,6] led to a systemic deviation towards an underestimation of Wmax, getting worse with increasing 6MWD. In these three studies, gender was not part of the equation despite having evaluated a mixed group. Holland et al. [28] also found considerable differences between three equations from literature estimating Wmax from 6MWD after applying them to 64 COPD patients; in men, the variation reached 47%. Besides the above-mentioned limited reproducibility of the applied methods, diverse protocols for cycle ergometer testing and inclusion of different predictors may also contribute to the identified differences. Only one equation from the literature [8] included a lung function parameter. Pretto et al. [29] did not use the 6MWD but baseline respiratory function as the only predictor of Wmax; the correlation was 0.85. This underlines the importance to include measures of lung function impairment when predicting Wmax. The equation for lung fibrosis [6] has been assessed in an Asian population and included FVC as an additional predictor. The application to our data led to residuals which showed a systematic deviation toward lower values independent of the 6MWD value, probably caused by different anthropometry.
Limitations of the Study
We included asthma patients who exhibited Wmax >200 W. In these patients, the 6MWD might not be an appropriate test. Due to the ceiling effect of the walking distance, the slope of the prediction equation could be calculated higher than in a more physically impaired cohort.
When comparing different prediction equations, the underlying study protocols and inclusion criteria may differ and comparability may be hampered. In addition, the specific populations have to be taken into account. Three equations were assessed in Australia and the Netherlands, countries with populations that can be regarded similar to German anthropometry. In the case of the other two equations, based on a Brazilian and an Asian population, the value of a comparison to a European population is limited because the anthropometric characteristics of the patients may differ considerably. This could lead to a different degree of correlation between the analyzed parameters, as well as to divergent slope and intercept of the regression line of the prediction equation.
Conclusion
Our results confirm the possibility to predict Wmax from 6MWD and related measures, but the achievable accuracy of prediction in single individuals is too low to be of practical value. A replacement of the individual assessment of Wmax by other tests cannot be advised.
Despite this, the prediction equations which we established on the basis of a large, heterogeneous data set might be useful for the estimation of average values in epidemiological and clinical studies, when cohorts in which Wmax has either been or not been determined are to be compared.
Acknowledgements
This study was supported and approved by the DGUV, German Social Accident Insurance (registered umbrella association of the accident insurance institutions for the industrial and public sectors).





![Fig. 3. a-f Residuals of predicted Wmax of COPD patients in comparison to residuals of other equations in the literature applied to the data set. a Residuals of data from this study according to equation (a) (see Results) in the obstructive group (n = 186) versus the 6MWD. b-f Residuals, having applied equations from references [4,5,6,7,8] to the data from this study for the obstructive group (n = 186) versus the 6MWD. b 103.217 + 30.500 (= male gender) + [-1.613·age (years) + (0.002·6MWW [km·kg])] [7]. c -51.994 - 0.505 (= male gender) - [0.234·age (years)] + [0.091·height (cm)] + [0.132·6MWD (m)] [8]. d 17.393 + [1.442·6MWW (km·kg)] [5]. e 2.310·6MWW (km·kg) + 8.820 [6]. f -27.9717 + [3.7792·6MWFFM (km·kg)] [4].](https://karger.silverchair-cdn.com/karger/content_public/journal/res/86/5/10.1159_000345859/2/m_000345859_f03.gif?Expires=1716367358&Signature=A7--um5eBhTnCtDJ-OVKRDcmSLkcXIKh7dvySHu0qd8stOVYEeer5Sk2kMveoDhwitoSwgbZ3N8cm9F8FX3Dix0NKBqbsCcWmnJF7YSVWDDQcFrQ4uhOfP~x1LAIcvw~2iUuNmbaORJ1Q6oINid9EjDTVw92XJykLY4DWSHQGdSgw18V7Q3Ix57zsF55duYWAqw9QtrRJnDRFU-ajxgpKHedvIGcTXMpw5XpMmfIpmkYiqVhyi3Eu0ffQIb6a1zfPBGN96i3V~IpmCDjHShkPCqPVLzIEibVORU6VQiExFhZ4HxbuYjS0R9CHpSsWFevv0hyqIqPIabFaLyNI9JcCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. a, b Residuals of predicted Wmax of asbestosis patients in comparison to residuals of the equation in the literature for idiopathic pulmonary fibrosis patients applied to the data set. a Residuals of the present data according to equation (e) (see Results) in the asbestosis group (n = 69) versus the 6MWD. b Residuals according to the equation from reference [6] (0.122·6MWD) + (0.387·FVC %pred.) - 21.474 applied to our own data, for the asbestosis group (n = 69), versus the 6MWD.](https://karger.silverchair-cdn.com/karger/content_public/journal/res/86/5/10.1159_000345859/2/m_000345859_f04.gif?Expires=1716367358&Signature=N8-11ZAcAVnQq8pTp3ODgZm7SgoPbeqCsqz8ibH-1CEU5xd2wath1mW7Z75NTRShwS0mcrFTeAvb2oL4s~HVMDd~HtwKWsgdRhO~onn1wHZUj~e9qAm6aPuRmvGdaCytMEcfddvQmAtOTxfwvEboBkkYA1f1ycySMPwpiJkT7xotw-T0oxXOKlp00H1uUHsjhIXFoFp9X54uR7rROvmkZRBHBEvlZgYlT3d7odcaG7-v1H-KSjCgU9pMjRvpg9HfVEzmiK3ra9hffKjf4DEp-9UZYzlSPEZPos1NPXzJLfQtoQhinXiYIUnT9x9mokU0WO8QRonBIPwQNsr~ET8uPg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)